IEC 61010-2-101:2015
(Main)Safety requirements for electrical equipment for measurement, control and laboratory use - Part 2-101: Particular requirements for in vitro diagnostic (IVD) medical equipment
Safety requirements for electrical equipment for measurement, control and laboratory use - Part 2-101: Particular requirements for in vitro diagnostic (IVD) medical equipment
IEC 61010-2-101:2015 applies to equipment intended for in vitro diagnostic (IVD) medical purposes, including self-test IVD medical purposes. It has the status of a group safety function, as specified in IEC Guide 104. This standard has been prepared in close collaboration with Working Group CENELEC BTTF 88.1. This second edition cancels and replaces the first edition published in 2002. It constitutes a technical revision and includes the following significant changes from the first edition, as well as numerous other changes:
- excluded IEC 61010-2-081 (general laboratory equipment) from the scope. This separates IEC 61010-2-081 and IEC 61010-2-101 equipment;
- updated Biohazard and Lot symbols in Table 1 in Clause 5;
- added requirement for within expiration consumables and authorized representative details in Instructions for Use to Clause 5;
- added requirement for gas or liquid markings and ratings to Clause 5;
- added requirement to include OPERATOR instructions to deal with consumable or sample spills, jams or breakage inside equipment, disposal of hazardous waste, personal protection, RISK reduction procedures relating to flammable liquids, burns from surfaces, and loading and unloading of sample and reagents in Instructions for Use to Clause 5;
- added requirement for manufacturer to provide instructions on equipment transport, storage and removal from use to Clause 5;
- added normative reference ISO 18113-5 for instructions for use of self-test IVD medical equipment in Clause 5;
- added requirement for OPERATOR maintenance instructions to Clause 7;
- added requirements for sample zones and loading zones to Clause 7;
- excluded equipment whose size and weight make unintentional movement unlikely from drop test in Clause 8;
- added requirement for biohazard marking to Clause 13;
- added requirement for interlock systems containing electric/electronic or programmable components to Clause 15;
- added informative reference to Usability standard IEC 62366 to Clause 16;
- replaced Clause 17 with requirements of ISO 14971 for RISK assessment.
- Annex BB Instructions for use for self-testing IVD Medical Equipment deleted and a reference given to ISO 18113-5 in Clause 5.
This publication is to be read in conjunction with IEC 61010-1:2010.
Règles de sécurité pour appareils électriques de mesurage, de régulation et de laboratoire - Partie 2-101: Exigences particulières pour les appareils médicaux de diagnostic in vitro (DIV)
IEC 61010-2-101:2015 s'applique aux appareils destinés aux applications médicales de diagnostic in vitro (DIV), y compris aux appareils médicaux d'autodiagnostic DIV. Cette norme a le statut de publication groupée de sécurité, conformément au Guide 104 de l'IEC. La préparation de cette norme a été réalisée en étroite collaboration avec le groupe de travail CENELEC BTTF 88.1. Cette deuxième édition annule et remplace la première édition parue en 2002. Cette édition constitue une révision technique et inclut les modifications techniques majeures suivantes par rapport à la première édition, ainsi que de nombreuses autres modifications:
- exclusion de l'IEC 61010-2-081 (appareils d'usage général de laboratoire) du domaine d'application, ce qui distingue les appareils de l'IEC 61010-2-081 et ceux de l'IEC 61010-2-101;
- mise à jour des symboles Danger biologique et Lot dans le Tableau 1 à l'Article 5;
- ajout d'une exigence relative aux consommables possédant une date d'expiration et aux informations concernant le représentant autorisé dans les Instructions d'utilisation à l'Article 5;
- ajout d'une exigence relative aux marquages et caractéristiques assignées des gaz et liquides à l'Article 5;
- ajout d'une exigence incluant des instructions à l'OPÉRATEUR permettant de couvrir les déversements, bourrages ou bris de consommables ou de prélèvements à l'intérieur des appareils, l'élimination des déchets dangereux, la protection individuelle, les procédures de réduction de RISQUE applicables aux liquides inflammables, brûlures causées par des surfaces, ainsi que le chargement et le déchargement de prélèvements et de réactifs dans les Instructions d'utilisation à l'Article 5;
- ajout d'une exigence imposant au fabricant de fournir des instructions relatives au transport, au stockage et au retrait d'utilisation des appareils à l'Article 5;
- ajout de la référence normative ISO 18113-5 relative aux instructions d'utilisation des appareils médicaux d'autodiagnostic DIV à l'Article 5;
- ajout d'exigences relatives aux instructions d'entretien par l'OPÉRATEUR à l'Article 7;
- ajout d'exigences relatives aux zones de prélèvement et aux zones de chargement à l'Article 7;
- exclusion des appareils dont la taille et le poids rendent improbable un mouvement involontaire de l'essai de chute à l'Article 8;
- ajout d'une exigence relative au marquage des dangers biologiques à l'Article 13;
- ajout d'une exigence relative aux systèmes de verrouillage incluant des composants électriques/électroniques ou programmables à l'Article 15;
- ajout d'une référence informative à la Norme d'aptitude à l'utilisation IEC 62366 à l'Article 16;
- remplacement de l'Article 17 par les exigences de l'ISO 14971 concernant l'évaluation du RISQUE.
- suppression des instructions d'utilisation de l'Annexe BB relatives aux appareils médicaux d'autodiagnostic DIV et ajout d'une référence à l'ISO 18113-5 à l'Article 5.
Cette publication doit être lue conjointement avec la CEI 61010-1:2010.
General Information
- Status
- Published
- Publication Date
- 22-Jan-2015
- Technical Committee
- TC 66 - Safety of measuring, control and laboratory equipment
- Drafting Committee
- MT 10 - TC 66/MT 10
- Current Stage
- DELPUB - Deleted Publication
- Start Date
- 05-Oct-2018
- Completion Date
- 30-Jun-2017
Relations
- Effective Date
- 05-Sep-2023
- Effective Date
- 05-Sep-2023
Overview
IEC 61010-2-101:2015 - "Safety requirements for electrical equipment for measurement, control and laboratory use - Part 2-101: Particular requirements for in vitro diagnostic (IVD) medical equipment" - is a Part 2 group safety standard that supplements IEC 61010-1:2010. It applies to electrical equipment intended for in vitro diagnostic (IVD) medical purposes, including self-test IVD devices used by lay persons. The 2015 second edition is a technical revision of the 2002 edition and clarifies safety, marking and documentation, mechanical resistance and risk-management expectations for IVD equipment.
Key topics and requirements
This standard focuses on practical safety controls and documentation for IVD medical equipment. Major topics include:
- Scope and application: Equipment intended for examination of specimens (blood, tissue) for diagnostics, monitoring or compatibility testing; self-test devices are explicitly included.
- Marking and documentation: Expanded requirements for Instructions for Use (IFU) - including authorized representative details, consumable expiration guidance, gas/liquid markings and ratings, and operator procedures for spills, jams, breakage, hazardous waste disposal and personal protection.
- Biohazard and lot symbols: Updated symbols and a reinforced requirement for biohazard marking where applicable.
- Risk management: Clause 17 replaced to adopt ISO 14971 requirements for medical device risk assessment; Annex AA supports risk management guidance.
- Operator and maintenance: Added requirements for operator maintenance instructions and inclusion of sample zones and loading zones for safe handling.
- Mechanical and environmental tests: Clarified drop test exclusions for very large/heavy equipment and requirements for mechanical resistance and heat/fire protection.
- Interlocks and electronic safety: Added requirements for interlock systems containing electric/electronic or programmable components to ensure safe operation.
- References to usability and labelling standards: Informative reference to IEC 62366 (usability) and normative reference to ISO 18113-5 for IFU of self-test IVDs.
Applications and who uses it
IEC 61010-2-101:2015 is essential for:
- Manufacturers of IVD analyzers, benchtop diagnostic instruments, point‑of‑care and self-test devices - for design, testing and documentation.
- Regulatory and compliance engineers, product safety teams and quality managers preparing conformity documentation or CE marking in jurisdictions referencing IEC/ISO standards.
- Notified bodies and test laboratories assessing electrical safety, risk management and usability aspects of IVD equipment.
- Procurement and clinical engineers evaluating safe deployment and maintenance procedures for laboratory and point-of-care diagnostics.
Related standards
- IEC 61010-1:2010 (general requirements) - read in conjunction with this Part 2.
- ISO 14971 - medical device risk management (adopted in Clause 17 replacement).
- ISO 18113-5 - labelling and instructions for self-test IVD medical devices.
- IEC 62366 - usability engineering (informative).
- IEC 61010-2-081 - general laboratory equipment (explicitly excluded from this Part 2 scope).
Keywords: IEC 61010-2-101:2015, IVD medical equipment safety, in vitro diagnostic standards, IEC 61010, ISO 14971, ISO 18113-5, biohazard marking, Instructions for Use.
Buy Documents
REDLINE IEC 61010-2-101:2015 - Safety requirements for electrical equipment for measurement, control and laboratory use – Part 2-101: Particular requirements for in vitro diagnostic (IVD) medical equipment Released:1/23/2015 Isbn:9782832222423
IEC 61010-2-101:2015 - Safety requirements for electrical equipment for measurement, control and laboratory use - Part 2-101: Particular requirements for in vitro diagnostic (IVD) medical equipment Released:1/23/2015 Isbn:9782832222065
Get Certified
Connect with accredited certification bodies for this standard

BSI Group
BSI (British Standards Institution) is the business standards company that helps organizations make excellence a habit.

TÜV Rheinland
TÜV Rheinland is a leading international provider of technical services.

TÜV SÜD
TÜV SÜD is a trusted partner of choice for safety, security and sustainability solutions.
Sponsored listings
Frequently Asked Questions
IEC 61010-2-101:2015 is a standard published by the International Electrotechnical Commission (IEC). Its full title is "Safety requirements for electrical equipment for measurement, control and laboratory use - Part 2-101: Particular requirements for in vitro diagnostic (IVD) medical equipment". This standard covers: IEC 61010-2-101:2015 applies to equipment intended for in vitro diagnostic (IVD) medical purposes, including self-test IVD medical purposes. It has the status of a group safety function, as specified in IEC Guide 104. This standard has been prepared in close collaboration with Working Group CENELEC BTTF 88.1. This second edition cancels and replaces the first edition published in 2002. It constitutes a technical revision and includes the following significant changes from the first edition, as well as numerous other changes: - excluded IEC 61010-2-081 (general laboratory equipment) from the scope. This separates IEC 61010-2-081 and IEC 61010-2-101 equipment; - updated Biohazard and Lot symbols in Table 1 in Clause 5; - added requirement for within expiration consumables and authorized representative details in Instructions for Use to Clause 5; - added requirement for gas or liquid markings and ratings to Clause 5; - added requirement to include OPERATOR instructions to deal with consumable or sample spills, jams or breakage inside equipment, disposal of hazardous waste, personal protection, RISK reduction procedures relating to flammable liquids, burns from surfaces, and loading and unloading of sample and reagents in Instructions for Use to Clause 5; - added requirement for manufacturer to provide instructions on equipment transport, storage and removal from use to Clause 5; - added normative reference ISO 18113-5 for instructions for use of self-test IVD medical equipment in Clause 5; - added requirement for OPERATOR maintenance instructions to Clause 7; - added requirements for sample zones and loading zones to Clause 7; - excluded equipment whose size and weight make unintentional movement unlikely from drop test in Clause 8; - added requirement for biohazard marking to Clause 13; - added requirement for interlock systems containing electric/electronic or programmable components to Clause 15; - added informative reference to Usability standard IEC 62366 to Clause 16; - replaced Clause 17 with requirements of ISO 14971 for RISK assessment. - Annex BB Instructions for use for self-testing IVD Medical Equipment deleted and a reference given to ISO 18113-5 in Clause 5. This publication is to be read in conjunction with IEC 61010-1:2010.
IEC 61010-2-101:2015 applies to equipment intended for in vitro diagnostic (IVD) medical purposes, including self-test IVD medical purposes. It has the status of a group safety function, as specified in IEC Guide 104. This standard has been prepared in close collaboration with Working Group CENELEC BTTF 88.1. This second edition cancels and replaces the first edition published in 2002. It constitutes a technical revision and includes the following significant changes from the first edition, as well as numerous other changes: - excluded IEC 61010-2-081 (general laboratory equipment) from the scope. This separates IEC 61010-2-081 and IEC 61010-2-101 equipment; - updated Biohazard and Lot symbols in Table 1 in Clause 5; - added requirement for within expiration consumables and authorized representative details in Instructions for Use to Clause 5; - added requirement for gas or liquid markings and ratings to Clause 5; - added requirement to include OPERATOR instructions to deal with consumable or sample spills, jams or breakage inside equipment, disposal of hazardous waste, personal protection, RISK reduction procedures relating to flammable liquids, burns from surfaces, and loading and unloading of sample and reagents in Instructions for Use to Clause 5; - added requirement for manufacturer to provide instructions on equipment transport, storage and removal from use to Clause 5; - added normative reference ISO 18113-5 for instructions for use of self-test IVD medical equipment in Clause 5; - added requirement for OPERATOR maintenance instructions to Clause 7; - added requirements for sample zones and loading zones to Clause 7; - excluded equipment whose size and weight make unintentional movement unlikely from drop test in Clause 8; - added requirement for biohazard marking to Clause 13; - added requirement for interlock systems containing electric/electronic or programmable components to Clause 15; - added informative reference to Usability standard IEC 62366 to Clause 16; - replaced Clause 17 with requirements of ISO 14971 for RISK assessment. - Annex BB Instructions for use for self-testing IVD Medical Equipment deleted and a reference given to ISO 18113-5 in Clause 5. This publication is to be read in conjunction with IEC 61010-1:2010.
IEC 61010-2-101:2015 is classified under the following ICS (International Classification for Standards) categories: 11.040.55 - Diagnostic equipment; 19.080 - Electrical and electronic testing. The ICS classification helps identify the subject area and facilitates finding related standards.
IEC 61010-2-101:2015 has the following relationships with other standards: It is inter standard links to IEC 61010-2-101:2002, IEC 61010-2-101:2018. Understanding these relationships helps ensure you are using the most current and applicable version of the standard.
IEC 61010-2-101:2015 is available in PDF format for immediate download after purchase. The document can be added to your cart and obtained through the secure checkout process. Digital delivery ensures instant access to the complete standard document.
Standards Content (Sample)
IEC 61010-2-101 ®
Edition 2.0 2015-01
REDLINE VERSION
INTERNATIONAL
STANDARD
colour
inside
GROUP SAFETY PUBLICATION
Safety requirements for electrical equipment for measurement, control and
laboratory use –
Part 2-101: Particular requirements for in vitro diagnostic (IVD) medical
equipment
All rights reserved. Unless otherwise specified, no part of this publication may be reproduced or utilized in any form
or by any means, electronic or mechanical, including photocopying and microfilm, without permission in writing from
either IEC or IEC's member National Committee in the country of the requester. If you have any questions about IEC
copyright or have an enquiry about obtaining additional rights to this publication, please contact the address below or
your local IEC member National Committee for further information.
IEC Central Office Tel.: +41 22 919 02 11
3, rue de Varembé Fax: +41 22 919 03 00
CH-1211 Geneva 20 info@iec.ch
Switzerland www.iec.ch
About the IEC
The International Electrotechnical Commission (IEC) is the leading global organization that prepares and publishes
International Standards for all electrical, electronic and related technologies.
About IEC publications
The technical content of IEC publications is kept under constant review by the IEC. Please make sure that you have the
latest edition, a corrigenda or an amendment might have been published.
IEC Catalogue - webstore.iec.ch/catalogue Electropedia - www.electropedia.org
The stand-alone application for consulting the entire The world's leading online dictionary of electronic and
bibliographical information on IEC International Standards, electrical terms containing more than 30 000 terms and
Technical Specifications, Technical Reports and other definitions in English and French, with equivalent terms in 15
documents. Available for PC, Mac OS, Android Tablets and additional languages. Also known as the International
iPad. Electrotechnical Vocabulary (IEV) online.
IEC publications search - www.iec.ch/searchpub IEC Glossary - std.iec.ch/glossary
The advanced search enables to find IEC publications by a More than 60 000 electrotechnical terminology entries in
variety of criteria (reference number, text, technical English and French extracted from the Terms and Definitions
committee,…). It also gives information on projects, replaced clause of IEC publications issued since 2002. Some entries
and withdrawn publications. have been collected from earlier publications of IEC TC 37,
77, 86 and CISPR.
IEC Just Published - webstore.iec.ch/justpublished
Stay up to date on all new IEC publications. Just Published IEC Customer Service Centre - webstore.iec.ch/csc
details all new publications released. Available online and If you wish to give us your feedback on this publication or
also once a month by email. need further assistance, please contact the Customer Service
Centre: csc@iec.ch.
IEC 61010-2-101 ®
Edition 2.0 2015-01
REDLINE VERSION
INTERNATIONAL
STANDARD
colour
inside
GROUP SAFETY PUBLICATION
Safety requirements for electrical equipment for measurement, control and
laboratory use –
Part 2-101: Particular requirements for in vitro diagnostic (IVD) medical
equipment
INTERNATIONAL
ELECTROTECHNICAL
COMMISSION
ICS 11.040.55, 19.080 ISBN 978-2-8322-2242-3
– 2 – IEC 61010-2-101:2015 RLV © IEC 2015
CONTENTS
FOREWORD . 3
1 Scope and object . 6
2 Normative references . 7
3 Terms and definitions . 7
4 Tests . 9
5 Marking and documentation . 9
6 Protection against electric shock . 14
7 Protection against mechanical HAZARDS . 14
8 Mechanical resistance to shock and impact Resistance to mechanical stresses . 16
9 Protection against the spread of fire . 17
10 Equipment temperature limits and resistance to heat . 17
11 Protection against HAZARDS from fluids . 17
12 Protection against radiation, including laser sources, and against sonic and
ultrasonic pressure . 17
13 Protection against liberated gases and substances, explosion and implosion . 17
14 Components and subassemblies . 18
15 Protection by interlocks . 18
16 Measuring circuits HAZARDS resulting from application . 19
17 RISK assessment . 19
Annexes . 19
Annex H L (informative) Index of defined terms . 19
Annex AA (normative) Risk management .
Annex BB (normative) Instructions for use for self-test IVD medical equipment .
Bibliography . 26
Table 1 – Symbols . 9
INTERNATIONAL ELECTROTECHNICAL COMMISSION
____________
SAFETY REQUIREMENTS FOR ELECTRICAL EQUIPMENT
FOR MEASUREMENT, CONTROL AND LABORATORY USE –
Part 2-101: Particular requirements for
in vitro diagnostic (IVD) medical equipment
FOREWORD
1) The International Electrotechnical Commission (IEC) is a worldwide organization for standardization comprising
all national electrotechnical committees (IEC National Committees). The object of IEC is to promote
international co-operation on all questions concerning standardization in the electrical and electronic fields. To
this end and in addition to other activities, IEC publishes International Standards, Technical Specifications,
Technical Reports, Publicly Available Specifications (PAS) and Guides (hereafter referred to as “IEC
Publication(s)”). Their preparation is entrusted to technical committees; any IEC National Committee interested
in the subject dealt with may participate in this preparatory work. International, governmental and non-
governmental organizations liaising with the IEC also participate in this preparation. IEC collaborates closely
with the International Organization for Standardization (ISO) in accordance with conditions determined by
agreement between the two organizations.
2) The formal decisions or agreements of IEC on technical matters express, as nearly as possible, an international
consensus of opinion on the relevant subjects since each technical committee has representation from all
interested IEC National Committees.
3) IEC Publications have the form of recommendations for international use and are accepted by IEC National
Committees in that sense. While all reasonable efforts are made to ensure that the technical content of IEC
Publications is accurate, IEC cannot be held responsible for the way in which they are used or for any
misinterpretation by any end user.
4) In order to promote international uniformity, IEC National Committees undertake to apply IEC Publications
transparently to the maximum extent possible in their national and regional publications. Any divergence
between any IEC Publication and the corresponding national or regional publication shall be clearly indicated in
the latter.
5) IEC itself does not provide any attestation of conformity. Independent certification bodies provide conformity
assessment services and, in some areas, access to IEC marks of conformity. IEC is not responsible for any
services carried out by independent certification bodies.
6) All users should ensure that they have the latest edition of this publication.
7) No liability shall attach to IEC or its directors, employees, servants or agents including individual experts and
members of its technical committees and IEC National Committees for any personal injury, property damage or
other damage of any nature whatsoever, whether direct or indirect, or for costs (including legal fees) and
expenses arising out of the publication, use of, or reliance upon, this IEC Publication or any other IEC
Publications.
8) Attention is drawn to the Normative references cited in this publication. Use of the referenced publications is
indispensable for the correct application of this publication.
9) Attention is drawn to the possibility that some of the elements of this IEC Publication may be the subject of
patent rights. IEC shall not be held responsible for identifying any or all such patent rights
This redline version of the official IEC Standard allows the user to identify the changes
made to the previous edition. A vertical bar appears in the margin wherever a change
has been made. Additions are in green text, deletions are in strikethrough red text.
– 4 – IEC 61010-2-101:2015 RLV © IEC 2015
International Standard IEC 61010-2-101 has been prepared by IEC technical committee 66:
Safety of measuring, control and laboratory equipment.
It has the status of a group safety publication, as specified in IEC Guide 104.
This standard has been prepared in close collaboration with Working Group CENELEC
BTTF 88.1.
This second edition cancels and replaces the first edition published in 2002. It constitutes a
technical revision and includes the following significant changes from the first edition, as well
as numerous other changes:
• excluded IEC 61010-2-081 (general laboratory equipment) from the scope. This separates
IEC 61010-2-081 and IEC 61010-2-101 equipment;
• updated Biohazard and Lot symbols in Table 1 in Clause 5;
• added requirement for within expiration consumables and authorized representative
details in Instructions for Use to Clause 5;
• added requirement for gas or liquid markings and ratings to Clause 5;
• added requirement to include OPERATOR instructions to deal with consumable or sample
spills, jams or breakage inside equipment, disposal of hazardous waste, personal
protection, RISK reduction procedures relating to flammable liquids, burns from surfaces,
and loading and unloading of sample and reagents in Instructions for Use to Clause 5;
• added requirement for manufacturer to provide instructions on equipment transport,
storage and removal from use to Clause 5;
• added normative reference ISO 18113-5 for instructions for use of self-test IVD medical
equipment in Clause 5;
• added requirement for OPERATOR maintenance instructions to Clause 7;
• added requirements for sample zones and loading zones to Clause 7;
• excluded equipment whose size and weight make unintentional movement unlikely from
drop test in Clause 8;
• added requirement for biohazard marking to Clause 13;
• added requirement for interlock systems containing electric/electronic or programmable
components to Clause 15;
• added informative reference to Usability standard IEC 62366 to Clause 16;
• replaced Clause 17 with requirements of ISO 14971 for RISK assessment.
• Annex BB Instructions for use for self-testing IVD Medical Equipment deleted and a
reference given to ISO 18113-5 in Clause 5.
The text of this standard is based on the following documents:
FDIS Report on voting
66/545/FDIS 66/560/RVD
Full information on the voting for the approval of this standard can be found in the report on
voting indicated in the above table.
This publication has been drafted in accordance with the ISO/IEC Directives, Part 2.
A list of all parts of the IEC 61010 series, under the general title: Safety requirements for
electrical equipment for measurement, control, and laboratory use, may be found on the IEC
website.
This Part 2-101 is intended to be used in conjunction with IEC 61010-1. It was established on
the basis of the third edition (2010).
This Part 2-101 supplements or modifies the corresponding clauses in IEC 61010-1 so as to
convert that publication into the IEC standard: Safety requirements for in vitro diagnostic
(IVD) medical equipment.
Where a particular subclause of Part 1 is not mentioned in this Part 2, that subclause applies
as far as is reasonable. Where this part states “addition”, “modification”, “replacement”, or
“deletion” the relevant requirement, test specification or note in Part 1 should be adapted
accordingly.
In this standard:
1) the following print types are used:
– requirements: in roman type;
– NOTES: in smaller roman type;
– conformity and test: in italic type;
– terms used throughout this standard which have been defined in clause 3: SMALL
ROMAN CAPITALS;
2) subclauses, figures, tables and notes which are additional to those in part 1 are numbered
starting from 101. Additional annexes are lettered starting from AA.
The committee has decided that the contents of this publication will remain unchanged until
the stability date indicated on the IEC web site under "http://webstore.iec.ch" in the data
related to the specific publication. At this date, the publication will be
• reconfirmed,
• withdrawn,
• replaced by a revised edition, or
• amended.
IMPORTANT – The “colour inside” logo on the cover page of this publication indicates
that it contains colours which are considered to be useful for the correct understanding
of its contents. Users should therefore print this publication using a colour printer.
– 6 – IEC 61010-2-101:2015 RLV © IEC 2015
SAFETY REQUIREMENTS FOR ELECTRICAL EQUIPMENT
FOR MEASUREMENT, CONTROL AND LABORATORY USE –
Part 2-101: Particular requirements for
in vitro diagnostic (IVD) medical equipment
1 Scope and object
This clause of Part 1 is applicable except as follows:
1.1.1 Equipment included in scope
Replacement:
Replace the text by the following:
This part of IEC 61010 applies to equipment intended for in vitro diagnostic (IVD) medical
purposes, including self-test IVD medical purposes.
IVD medical equipment, whether used alone or in combination, is intended by the
manufacturer to be used in vitro for the examination of specimens, including blood and tissue
samples, derived from the human body, solely or principally for the purpose of providing
information concerning one or more of the following:
• a physiological or pathological state; or
• a congenital abnormality;
• the determination of safety and compatibility with potential recipients;
• the monitoring of therapeutic measures.
Self-test IVD medical equipment is intended by the manufacturer for use by lay persons in a
home environment.
NOTE If all or part of the equipment falls within the scope of one or more other part 2 standards of IEC 61010 as
well as within the scope of this standard, it will also need to meet the requirements of considerations have to be
given to those other part 2 standards.
1.1.2 Equipment excluded from scope
Addition:
Add the following item:
aa) Products for general laboratory use are not IVD medical devices unless such products,
in view of their characteristics, Equipment in the scope of IEC 61010-2-081 unless they
are specifically intended by their manufacturer to be used for in vitro diagnostic
examination.
1.2 Object
1.2.1 Aspects included in scope
Replacement:
Replace the first sentence by the following:
The purpose of the requirements of this standard is to ensure that the design and the
methods of construction used provide a high degree of protection at a TOLERABLE RISK for
the OPERATOR and the surrounding area, using RISK management where appropriate (see
annex AA).
Addition:
Add two items:
h aa) biohazards;
i bb) hazardous chemical substances.
1.2.2 Aspects excluded from scope
Addition:
Add the following item and note:
g aa) the handling or manipulation outside the equipment of material under analysis.
NOTE Requirements covering these subjects are the responsibility of committees preparing relevant standards.
2 Normative references
This clause of Part 1 is applicable except as follows:
Addition:
Add the following references:
ISO 14971:2000, Medical devices – Application of risk management to medical devices
ISO 18113-5, In vitro diagnostic medical devices – Information supplied by the manufacturer
(labelling) – In vitro diagnostic instruments for selftesting
ISO 13857, Safety of machinery – Safety distances to prevent hazard zones being reached by
upper and lower limbs
3 Terms and definitions
This clause of Part 1 is applicable except as follows:
3.1 Equipment and states of equipment
Addition:
Add the following terms and definitions:
– 8 – IEC 61010-2-101:2015 RLV © IEC 2015
3.101
HARM
physical injury or damage to the health of people, or damage to property or the environment
[ISO/IEC Guide 51:1999, definition 3.3]
3.102
RISK
combination of the probability of occurrence of HARM and the severity of that HARM
[ISO/IEC Guide 51:1999, definition 3.2]
3.103
TOLERABLE RISK
RISK which is accepted in a given context based on the current values of society
[ISO/IEC Guide 51:1999, definition 3.7]
NOTE 1 TOLERABLE RISK is the result of a balance between the ideal of absolute safety, the demands to be met by
a product, process or service, and factors such as benefit to the user, suitability of purpose, cost effectiveness,
RISK evaluation, conventions of the society concerned, and the state of the art.
NOTE 2 The term “acceptable risk” is used in ISO 14971 in the same sense as TOLERABLE RISK.
3.104
REASONABLY FORESEEABLE MISUSE
use of a product, process or service in a way not intended by the supplier, but which may
result from readily predictable human behaviour
[ISO/IEC Guide 51:1999, definition 3.14]
3.105
PERMANENTLY AFFIXED
removable only with a TOOL or by appreciable force and able to withstand the effects of
temperature, rubbing, common solvents, reagents and vapours encountered during normal
use
3.106
MARKING
inscription, in writing or as a graphical symbol, PERMANENTLY AFFIXED to a product
3.1.101
SAMPLE ZONE
area where OPERATOR access is typically unintended; the inside of this zone presents
mechanical HAZARDS and a more likely probability of biohazardous human skin puncture
3.1.102
LOADING ZONE
area of automated equipment where an OPERATOR handles sample or reagent material.
3.5.12 RESPONSIBLE BODY
Addition:
Add the following note:
NOTE 1 This is not the European Community responsible authority.
4 Tests
This clause of Part 1 is applicable except as follows.
4.4.1 General
Replacement:
Replace item a) of the first paragraph by:
a) Equipment and circuit diagrams shall be examined to determine the fault con-
ditions which could arise in NORMAL USE and REASONABLY FORESEEABLE MISUSE, and
which could cause a HAZARD.
Deletion:
Delete the first dash.
Addition:
Additional subclause:
4.4.2.101 Incorrect voltage selection
Multivoltage equipment that can be set by the OPERATOR to different supply voltages shall be
set to each voltage in turn and then connected to all other RATED supply voltages in turn.
5 Marking and documentation
This clause of Part 1 is applicable except as follows:
5.1.1 General
Replacement:
Replace the third paragraph by the following:
Letter symbols for quantities and units shall be in accordance with IEC 60027. Internationally
recognized symbols, including those of Table 1, shall be used as far as possible. If other
additional symbols are required, it shall not be possible to confuse them with the
internationally recognized symbols. There are no colour requirements for symbols, except for
symbol 101 (see table 1). Graphic symbols shall be explained in the documentation.
Table 1 – Symbols
Addition:
Add the following symbols to Table 1:
– 10 – IEC 61010-2-101:2015 RLV © IEC 2015
Number Symbol Publication Description
Background colour
– yellow optional;
Biohazard
Symbol colour
101 ISO 7000- 0659 (2004-01)
Biological RISKS
– optional;
Outline / outline colour
– black optional;
EN 980, subclause 4
102 Batch code
ISO 7000- 2492 (2004-01)
5.1.2 Identification
Replacement:
Replace the text by the following:
Equipment shall, as a minimum, be marked with the following information:
a) manufacturer’s name or trade mark, and the address. The address shall include at least
the city and country;
NOTE 1 National regulation may require more details on the address than required in a).
b) model number, name, or other means of identifying the equipment;
c) where this is required by regulation, the name and address of the authorized represen-
tative of the manufacturer;
NOTE For example, in the EU this is the natural or legal person as established within the EC.
The following additional information shall be marked on the equipment or packaging or in the
instructions for use:
1) the serial number, for example SN XXXX or alternatively the batch code, preceded by
‘LOT’, using symbol 102 of Table 1;
2) the following information:
i) a clear indication that the equipment is IVD medical equipment;
ii) if applicable, a clear indication that the equipment is self-test IVD medical
equipment;
iii) if a potential RISK is posed, the identification of detachable components by
manufacturer and part identification, and where appropriate the batch code, etc.
iv) any expiry date of consumable parts, expressed as the year, the month and (where
relevant) the day, in that order.
3) instructions for use shall require that the OPERATOR only use consumables that are
within their expiration date. Where this is required by regulation, the name and address
of the authorized representative of the manufacturer.
NOTE 2 For example, in the European Union this is the natural or legal person as established within
the European Community.
5.1.5 TERMINALS, connections and operating devices
Addition:
Add the following subclause:
5.1.5.101 Gas and liquid connections
If necessary for safety, the equipment shall be clearly marked near to the connector on the
equipment with;
a) a means of identifying the gas or liquid to be used. Where no internationally recognized
symbol (including chemical formulae) exists, the equipment shall be marked with
symbol 14 of Table 1;
b) the maximum permitted pressure, or alternatively symbol 14 of Table 1 (see 5.4.3).
Conformity is checked by inspection.
Addition:
Add the following subclause:
5.1.101 Transport and storage
Packaging of equipment shall be labelled to indicate any special conditions for transport or
storage (see 5.4.102).
Conformity is checked by inspection.
5.2 Warning markings
Replacement:
Replace the fifth first paragraph by the following four paragraphs:
Equipment that can be potentially infectious due to the samples or reagents used shall be
prominently marked with symbol 101 of Table 1.
Equipment that can be hazardous due to the use of chemical substances shall be marked with
the appropriate symbol, or (if none is available) symbol 14 of Table 1.
Containers or bags for biohazardous waste material which can be removed from the
equipment during NORMAL USE shall be marked with symbol 101 of Table 1.’
Warning markings specified in 5.1.5.1, 5.1.5.2 c), 5.1.5.2 d), 5.1.5.101, 6.1.2 b), 6.5.1.2 g),
6.6.2, 7.2 c), 7.3.2 b) 3), 7.4, 10.1, 13.2.2 and 13.101 shall meet the following requirements.
5.3 Durability of markings
Replacement:
Replace the first paragraph by the following paragraph:
Markings required by 5.1.2 to 5.2 shall be PERMANENTLY AFFIXED and shall remain clear and
legible under conditions of NORMAL USE, and resist the effects of temperature and rubbing, and
of solvent and reagents likely to be encountered in NORMAL USE, including cleaning and
decontaminating agents specified by the manufacturer.
Addition:
Add after the first second paragraph the following paragraph:
– 12 – IEC 61010-2-101:2015 RLV © IEC 2015
If a solvent or reagent specified for use with the equipment could affect the durability of a
particular marking, that marking is also rubbed for 30 s with each the most frequently used
and/or aggressive solvent or reagent to which the equipment is likely to be exposed in NORMAL
USE
(or with A representative sample of groups of solvents or reagents likely to have a similar
effect can optional be used).
5.4.1 General
Deletion:
Delete the note 2 in the second paragraph.
Addition:
Add a new third paragraph as follows:
Information shall be given about any RISKS not reduced to a TOLERABLE RISK level by the
protective measures specified in this standard. If there is a need for training or for the use of
additional protective devices or personal protective equipment to reduce RISKS to a TOLERABLE
RISK level, these shall be specified.
5.4.3 Equipment installation
Replacement:
Replace subclause 5.4.3 by the following:
5.4.3 Equipment transportation, installation and assembly instructions
Documentation for the RESPONSIBLE BODY shall include the following if applicable:
a) instructions for transportation after delivery to the RESPONSIBLE BODY;
b) floor loading requirements;
NOTE Mass and dimensions are sufficient information for floor loading.
c) individual weights mass of principal heavy subassemblies units;
d) location and mounting instructions, including the space required for ventilation, and for
safe and efficient OPERATOR maintenance;
e) assembly instructions;
f) instructions for protective earthing;
g) the sound data required by 12.5.1;
h) instructions relating to the handling, containment and exhaust of hazardous substances,
including any requirements for preventing back-syphonage;
i) any drainage systems required where a HAZARD could occur from the discharge of
biological and chemical substances and hot fluids;
j) details of protective measures relating to hazardous radiation (see clause 12);
k) connections to the supply;
l) for PERMANENTLY CONNECTED EQUIPMENT only:
1) MAINS supply requirements and details of connections, including the RATED temperature
of the cable required at maximum RATED ambient temperature;
2) requirements for any external switch or circuit-breaker (see 6.11.2.1) and external
overcurrent protection devices (see 9.6.1) and a recommendation that the switch or
circuit-breaker be near the equipment if this is necessary for safety;
m) requirements for special services (for example air, cooling liquid) including pressure limits.
Conformity is checked by inspection of the documentation.
5.4.4 Equipment operation
Replacement:
Replace the first paragraph text by the following:
Instructions for use shall include if applicable:
a) details of operating controls and their use in all operating modes; with any sequence of
operation;
NOTE 1 IEC 60073 gives guidance on colours and symbols of operating controls.
b) an instruction not to position the equipment so that it is difficult to operate the
disconnecting device (see 6.11);
c) instructions for interconnections to accessories and other equipment, including details of
suitable accessories, detachable parts and any special consumable materials;
d) limits for intermittent operation;
e) an explanation of symbols used on the equipment and, where HAZARDS are involved, the
reason for using a symbol in each particular case;
f) instructions for any actions to be taken by an OPERATOR in case of a malfunction to deal
with a HAZARD resulting from equipment spills, lock-ups, container breakage and similar
malfunctions;
g) instructions and recommendations for cleaning and decontamination, with materials
recommended (see 11.2);
h) instructions for the disposal of hazardous waste;
i) if NORMAL USE involves the handling of hazardous chemical substances, instructions on
correct use and any need for training or personal protection measures;
j) Appropriate instruction to use personal protective equipment (e.g. gloves, gowns) where
there could be contact with the skin when handling potentially infectious substances or
surfaces (such as human samples or reagents), the need to use protective gloves or other
protective means;
k) Appropriate instructions and requirements for protection of the mouth, nose or eyes shall
be given where the equipment could emit hazardous aerosol vapours in NORMAL USE;
l) Appropriate instructions and requirements for protective devices, such as protective
glasses shall be given where potentially hazardous visible or invisible radiation could be
emitted;
m) a statement in the instructions that, if the equipment is used in a manner not specified by
the manufacturer, the protection provided by the equipment may be impaired.
detailed instructions about RISK reduction procedures relating to flammable liquids (see
9.5 c));
n) details of methods of reducing the RISKS of burns from surfaces permitted to exceed the
temperature limits of 10.1.
o) Appropriate warnings to reduce RISK during loading and unloading of samples and
reagents (see 7.3.102)
p) Instructions for the RESPONSIBLE BODY to ensure that all retaining hardware (e.g screws,
fasteners) are in place on removable PROTECTIVE BARRIERS, and the removable PROTECTIVE
BARRIERS are in place on the instrument during normal operation.
– 14 – IEC 61010-2-101:2015 RLV © IEC 2015
q) A statement that, if a TOOL is required to remove a fixed PROTECTIVE BARRIER and/or
ENCLOSURE guarding a SAMPLE ZONE, access to that tool should be controlled by the
RESPONSIBLE BODY.
r) A statement listing the tools to be controlled by the RESPONSIBLE BODY.
NOTE 2 Information on decontaminants their use, dilution and potential application is contained in the Laboratory
Biosafety Manual, published by the World Health Organization and the Biosafety in Microbiological and Biomedical
Laboratories, published by Centers for Disease Control and Prevention and National Institutes of Health,
Washington. There are also national guidelines that cover these areas.
NOTE 3 Cleaning and decontamination may be necessary as a safeguard when equipment and their accessories
are maintained, repaired, or transferred. Preferably manufacturers should provide a format for the RESPONSIBLE
BODY to certify to those maintaining, repairing or transferring equipment that such a treatment has been carried out.
Conformity is checked by inspection of the documentation.
Addition:
Add the following subclauses:
5.4.4.101 Instructions for use, self-test IVD medical equipment
Instructions for use of self-test IVD medical equipment are given in annex BB shall comply
with ISO 18113-5.
Conformity is checked by inspection of the documentation.
5.4.101 Removal of equipment from use for repair or disposal
Instructions shall be provided for the RESPONSIBLE BODY for eliminating or reducing HAZARDS
involved in removal from use, transportation or disposal. These instructions shall include
requirements for minimizing biohazards, or appropriate contact information shall be provided
in the documentation.
NOTE Regional or international requirements can apply.
Conformity is checked by inspection of the documentation.
5.4.102 Transport and storage
The manufacturer shall specify the conditions for transport and storage of the equipment. The
documentation shall contain a specification of the permissible environmental conditions for
transport and storage which. Essential information shall be repeated on the outside of the
packaging of the equipment (see 5.1) package using appropriate symbols (see 5.1.101).
When the manufacturer assumes responsibility for delivery and installation the above is not
required in the documentation.
Compliance is checked by inspection.
6 Protection against electric shock
This clause of part 1 is applicable.
7 Protection against mechanical HAZARDS
This clause of part 1 is applicable, except as follows:
7.3.1 General
Replacement:
Replace the second sentence as follows:
The conditions specified in 7.3.4, 7.3.5, and 7.3.101 are considered to represent a tolerable
level.
Replace the conformity statement as follows:
Conformity is checked as specified in 7.3.2, 7.3.3, 7.3.4, 7.3.5, 7.3.101, and Clause 17 as
applicable.
7.3.2 Exceptions
Replacement:
Replace item b) 3) text by the following:
There are warning markings prohibiting access by untrained OPERATORS. Markings shall be
placed within the area requiring maintenance where they can alert the OPERATOR to the
HAZARD. As an alternative, symbol 14 of Table 1 can be used, with the warnings included in
the documentation.
Addition:
Add the following item to to the list:
b) 4) There are OPERATOR maintenance instructions that specify safe maintenance
procedures.
7.3.3 RISK assessment for mechanical HAZARDS to body parts
Replacement:
Replace text by the following:
If equipment is specified by the manufacturer for continuous loading of sample and reagent
materials and associated HAZARDS in the SAMPLE ZONE are solely caused by the sample and/or
reagent probes 7.3.101 applies specifically for the SAMPLE ZONE. Subclause 7.3.101 does not
apply to self-testing and point of care equipment.
RISKS shall be reduced to a tolerable level by at least the applicable minimum protective
measure of Table 12, taking into account the severity, probability of exposure and possibility
of avoiding the HAZARD.
Conformity is checked by evaluation of the RISK assessment documentation to ensure that the
RISKS have been eliminated or that only TOLERABLE RISKS remain.
Table 12 – Protective measures against mechanical HAZARDS to body parts
Replacement:
Replace the text of item B by the following text:
Moderate measures; emergency switches, PROTECTIVE BARRIERS or covers removable only
with a TOOL, distances (see ISO 13857), or separations (see ISO 13854 or EN 349).
– 16 – IEC 61010-2-101:2015 RLV © IEC 2015
Addition:
Add the following subclause:
7.3.101 SAMPLE ZONE
Equipment with a SAMPLE ZONE shall comply with the requirements of one or more of the
following:
aa) PROTECTIVE BARRIER or
bb) all following measures apply:
1) The minimum maintained gap between LOADING ZONE and SAMPLE ZONE is 120 mm.
2) Unintentional contact between OPERATOR and sample/reagent pipettor is unlikely.
3) The area between LOADING ZONE and SAMPLE ZONE is marked with symbol 14 and
symbol 101 of table 1 (see 5.4.4 o)), or if not visible by the OPERATOR the marking shall
be located visible and close to the area.
8 Mechanical resistance to shock and impact Resistance to mechanical
stresses
This clause of part 1 is applicable except as follows:
Addition:
Additional subclause:
8.1 General
Replacement:
Replace the text of item 3) by the following:
3) except for FIXED EQUIPMENT, for equipment with a mass over 100 kg, or for equipment
whose size and weight make unintentional movement unlikely and which is not moved in
NORMAL USE, the appropriate test of 8.3. The equipment is not operated during the tests.
Addition:
Add the following subclause:
8.101 Transport and storage
When packed delivered in the manufacturer’s packaging, equipment shall not cause a HAZARD
during NORMAL USE after transport or storage in the conditions specified by the manufacturer
(see 5.1.101 and 5.4.101).
If the manufacturer assumes responsibility for delivery and installation, the above requirement
is met without inspection of test records.
Conformity is checked by inspection of records of transport tests performed by the
manufacturer.
NOTE Guidance on tests is given in ASTM D4169, and in the publications of the International Safe Transport
Association (ISTA).
9 Protection against the spread of fire
This clause of Part 1 is applicable.
10 Equipment temperature limits and resistance to heat
This clause of Part 1 is applicable.
11 Protection against HAZARDS from fluids
This clause of Part 1 is applicable except as follows:
11.3 Spillage
Replacement:
If in NORMAL USE liquid is likely to be spilled into the equipment, the equipment shall be
designed so that no HAZARD will occur, as a result of the wetting of insulation or of internal
uninsulated parts which are HAZARDOUS LIVE, or as a result of the contact of potentially
aggressive substances (such as corrosive, toxic or flammable liquids) with parts of the
equipment.
Conformity is checked by inspection. In case of doubt, 0,2 l of water is poured steadily from a
height of 0,1 m over a period of 15 s onto each point in turn at the area where the OPERATOR
has to pour in or handle liquids, and where the liquid might gain access to electrical parts.
Immediately after this treatment, the equipment shall pass the voltage tests of 6.8 (without
humidity preconditioning) and ACCESSIBLE parts shall not exceed the limits of 6.3.1.
Where appropriate, conformity is also checked by an examination of the compatibility of
potentially aggressive substances with contacted parts of the equipment.
12 Protection against radiation, including laser sources, and against sonic and
ultrasonic pressure
This clause of Part 1 is applicable.
13 Protection against liberated gases and substances, explosion and implosion
This clause of part 1 is applicable except as follows:
Modification:
Modify the title of the clause as follows:
13 Protection against liberated gases and substances, explosion and implosion
13.1 Poisonous and injurious gases
Modification:
Modify the title as follows:
– 18 – IEC 61010-2-101:2015 RLV © IEC 2015
13.1 Poisonous and injurious gases and substances
Replacement:
Replace the first paragraph by the following two new paragraphs:
Equipment shall not liberate dangerous amounts of poisonous or injurious gases or
substances in NORMAL CONDITION or in SINGLE FAULT CONDITION.
If potentially hazardous substances are used in the equipment, the OPERATOR shall not be
wetted nor be able to inhale quantities likely to be hazardous. The areas of the equipment
containing such substances shall be equipped with protective covers or similar means of
protection.
Addition:
Add the following subclause:
13.101 Biohazardous substances
Equipment that can be potentially infectious due to the samples or reagents used shall be
prominently marked with symbol 101 of Table 1. At minimum, a biohazard symbol shall be
near the sampling area and visible in NORMAL USE.
Biohazard symbols shall be near biohazardous areas accessed during OPERATOR maintenance
visible only during this maintenance.
Symbol 101 of Table 1 shall be marked on containers or bags for biohazardous waste material
which can be removed from the equipment during NORMAL USE, and near any biohazardous
drain connection.
Equipment that can be hazardous due to the use of hazardous substances shall be marked
with the appropriate international symbol, or (if none is available) symbol 14 of Table 1.
14 Components and subassemblies
This clause of Part 1 is applicable except as follows:
14.3 Over-temperature protection devices
Addition:
Add the following paragraph after the second paragraph:
Over-temperature protection devices in self-test IVD medical equipment shall not be self-
resetting.
15 Protection by interlocks
This clause of Part 1 is applicable except as follows.
15.1 General
Addition:
Add the following text after the first sentence:
As an alternative method, for interlock systems containing electric/electronic or programmable
components (E/E/P components) the reliability and design requirements can be determined by
applying e.g. IEC 62061 (SIL) or ISO 13849 (PL) or other solutions providing equivalent
functional safety.
16 Measuring circuits HAZARDS resulting from application
This clause of Part 1 is applicable except as follows:
16.2 Ergonomic aspects
Replacement:
Replace the note by the following note:
NOTE RISK assessment procedures for ergonomics can be found in IEC 62366, EN 894-2, EN 894-3, ISO 9241,
SEMI S8 and other documents. Not all of the requirements in these documents will be applicable to equipment
within the scope of this standard.
17 RISK assessment
This clause of Part 1 is replaced as follows:
Replacement:
RISK assessment shall be carried out and documented using the requirements of ISO 14971
for HAZARDS not addressed in this standard and Part 1.
Conformity is checked by evaluation of the RISK assessment documentation to assure that the
RISKS have been eliminated or that only TOLERABLE RISKS remain.
Annexes
The annexes of Part 1 are applicable except as follows:
Additions:
Annex H L
(informative)
Index of defined terms
Addition:
Add the following defined terms to the list:
HARM . 3.101
MARKING . 3.106
PERMANENTLY AFFIXED . 3.105
REASONABLY FORESEEABLE MISUSE . 3.104
RISK .
...
IEC 61010-2-101 ®
Edition 2.0 2015-01
INTERNATIONAL
STANDARD
NORME
INTERNATIONALE
GROUP SAFETY PUBLICATION
PUBLICATION GROUPÉE DE SÉCURITÉ
Safety requirements for electrical equipment for measurement, control and
laboratory use –
Part 2-101: Particular requirements for in vitro diagnostic (IVD) medical
equipment
Règles de sécurité pour appareils électriques de mesurage, de régulation et de
laboratoire –
Partie 2-101: Exigences particulières pour les appareils médicaux de diagnostic
in vitro (DIV)
All rights reserved. Unless otherwise specified, no part of this publication may be reproduced or utilized in any form
or by any means, electronic or mechanical, including photocopying and microfilm, without permission in writing from
either IEC or IEC's member National Committee in the country of the requester. If you have any questions about IEC
copyright or have an enquiry about obtaining additional rights to this publication, please contact the address below or
your local IEC member National Committee for further information.
Droits de reproduction réservés. Sauf indication contraire, aucune partie de cette publication ne peut être reproduite
ni utilisée sous quelque forme que ce soit et par aucun procédé, électronique ou mécanique, y compris la photocopie
et les microfilms, sans l'accord écrit de l'IEC ou du Comité national de l'IEC du pays du demandeur. Si vous avez des
questions sur le copyright de l'IEC ou si vous désirez obtenir des droits supplémentaires sur cette publication, utilisez
les coordonnées ci-après ou contactez le Comité national de l'IEC de votre pays de résidence.
IEC Central Office Tel.: +41 22 919 02 11
3, rue de Varembé Fax: +41 22 919 03 00
CH-1211 Geneva 20 info@iec.ch
Switzerland www.iec.ch
About the IEC
The International Electrotechnical Commission (IEC) is the leading global organization that prepares and publishes
International Standards for all electrical, electronic and related technologies.
About IEC publications
The technical content of IEC publications is kept under constant review by the IEC. Please make sure that you have the
latest edition, a corrigenda or an amendment might have been published.
IEC Catalogue - webstore.iec.ch/catalogue Electropedia - www.electropedia.org
The stand-alone application for consulting the entire The world's leading online dictionary of electronic and
bibliographical information on IEC International Standards, electrical terms containing more than 30 000 terms and
Technical Specifications, Technical Reports and other definitions in English and French, with equivalent terms in 15
documents. Available for PC, Mac OS, Android Tablets and additional languages. Also known as the International
iPad. Electrotechnical Vocabulary (IEV) online.
IEC publications search - www.iec.ch/searchpub IEC Glossary - std.iec.ch/glossary
The advanced search enables to find IEC publications by a More than 60 000 electrotechnical terminology entries in
variety of criteria (reference number, text, technical English and French extracted from the Terms and Definitions
committee,…). It also gives information on projects, replaced clause of IEC publications issued since 2002. Some entries
and withdrawn publications. have been collected from earlier publications of IEC TC 37,
77, 86 and CISPR.
IEC Just Published - webstore.iec.ch/justpublished
Stay up to date on all new IEC publications. Just Published IEC Customer Service Centre - webstore.iec.ch/csc
details all new publications released. Available online and If you wish to give us your feedback on this publication or
also once a month by email. need further assistance, please contact the Customer Service
Centre: csc@iec.ch.
A propos de l'IEC
La Commission Electrotechnique Internationale (IEC) est la première organisation mondiale qui élabore et publie des
Normes internationales pour tout ce qui a trait à l'électricité, à l'électronique et aux technologies apparentées.
A propos des publications IEC
Le contenu technique des publications IEC est constamment revu. Veuillez vous assurer que vous possédez l’édition la
plus récente, un corrigendum ou amendement peut avoir été publié.
Catalogue IEC - webstore.iec.ch/catalogue Electropedia - www.electropedia.org
Application autonome pour consulter tous les renseignements
Le premier dictionnaire en ligne de termes électroniques et
bibliographiques sur les Normes internationales,
électriques. Il contient plus de 30 000 termes et définitions en
Spécifications techniques, Rapports techniques et autres
anglais et en français, ainsi que les termes équivalents dans
documents de l'IEC. Disponible pour PC, Mac OS, tablettes
15 langues additionnelles. Egalement appelé Vocabulaire
Android et iPad.
Electrotechnique International (IEV) en ligne.
Recherche de publications IEC - www.iec.ch/searchpub
Glossaire IEC - std.iec.ch/glossary
La recherche avancée permet de trouver des publications IEC Plus de 60 000 entrées terminologiques électrotechniques, en
en utilisant différents critères (numéro de référence, texte, anglais et en français, extraites des articles Termes et
comité d’études,…). Elle donne aussi des informations sur les Définitions des publications IEC parues depuis 2002. Plus
projets et les publications remplacées ou retirées. certaines entrées antérieures extraites des publications des
CE 37, 77, 86 et CISPR de l'IEC.
IEC Just Published - webstore.iec.ch/justpublished
Service Clients - webstore.iec.ch/csc
Restez informé sur les nouvelles publications IEC. Just
Published détaille les nouvelles publications parues. Si vous désirez nous donner des commentaires sur cette
Disponible en ligne et aussi une fois par mois par email. publication ou si vous avez des questions contactez-nous:
csc@iec.ch.
IEC 61010-2-101 ®
Edition 2.0 2015-01
INTERNATIONAL
STANDARD
NORME
INTERNATIONALE
GROUP SAFETY PUBLICATION
PUBLICATION GROUPÉE DE SÉCURITÉ
Safety requirements for electrical equipment for measurement, control and
laboratory use –
Part 2-101: Particular requirements for in vitro diagnostic (IVD) medical
equipment
Règles de sécurité pour appareils électriques de mesurage, de régulation et de
laboratoire –
Partie 2-101: Exigences particulières pour les appareils médicaux de diagnostic
in vitro (DIV)
INTERNATIONAL
ELECTROTECHNICAL
COMMISSION
COMMISSION
ELECTROTECHNIQUE
INTERNATIONALE
ICS 11.040.55, 19.080 ISBN 978-2-8322-2206-5
– 2 – IEC 61010-2-101:2015 © IEC 2015
CONTENTS
FOREWORD . 3
1 Scope and object . 6
2 Normative references. 7
3 Terms and definitions . 7
4 Tests . 7
5 Marking and documentation . 8
6 Protection against electric shock . 12
7 Protection against mechanical HAZARDS . 12
8 Resistance to mechanical stresses . 14
9 Protection against the spread of fire . 14
10 Equipment temperature limits and resistance to heat . 14
11 Protection against HAZARDS from fluids . 14
12 Protection against radiation, including laser sources, and against sonic and
ultrasonic pressure . 14
13 Protection against liberated gases and substances, explosion and implosion . 15
14 Components and subassemblies . 15
15 Protection by interlocks . 15
16 HAZARDS resulting from application. 15
17 RISK assessment . 16
Annexes . 16
Annex L (informative) Index of defined terms . 17
Bibliography . 18
Table 1 – Symbols . 8
INTERNATIONAL ELECTROTECHNICAL COMMISSION
____________
SAFETY REQUIREMENTS FOR ELECTRICAL EQUIPMENT
FOR MEASUREMENT, CONTROL AND LABORATORY USE –
Part 2-101: Particular requirements for
in vitro diagnostic (IVD) medical equipment
FOREWORD
1) The International Electrotechnical Commission (IEC) is a worldwide organization for standardization comprising
all national electrotechnical committees (IEC National Committees). The object of IEC is to promote
international co-operation on all questions concerning standardization in the electrical and electronic fields. To
this end and in addition to other activities, IEC publishes International Standards, Technical Specifications,
Technical Reports, Publicly Available Specifications (PAS) and Guides (hereafter referred to as “IEC
Publication(s)”). Their preparation is entrusted to technical committees; any IEC National Committee interested
in the subject dealt with may participate in this preparatory work. International, governmental and non-
governmental organizations liaising with the IEC also participate in this preparation. IEC collaborates closely
with the International Organization for Standardization (ISO) in accordance with conditions determined by
agreement between the two organizations.
2) The formal decisions or agreements of IEC on technical matters express, as nearly as possible, an international
consensus of opinion on the relevant subjects since each technical committee has representation from all
interested IEC National Committees.
3) IEC Publications have the form of recommendations for international use and are accepted by IEC National
Committees in that sense. While all reasonable efforts are made to ensure that the technical content of IEC
Publications is accurate, IEC cannot be held responsible for the way in which they are used or for any
misinterpretation by any end user.
4) In order to promote international uniformity, IEC National Committees undertake to apply IEC Publications
transparently to the maximum extent possible in their national and regional publications. Any divergence
between any IEC Publication and the corresponding national or regional publication shall be clearly indicated in
the latter.
5) IEC itself does not provide any attestation of conformity. Independent certification bodies provide conformity
assessment services and, in some areas, access to IEC marks of conformity. IEC is not responsible for any
services carried out by independent certification bodies.
6) All users should ensure that they have the latest edition of this publication.
7) No liability shall attach to IEC or its directors, employees, servants or agents including individual experts and
members of its technical committees and IEC National Committees for any personal injury, property damage or
other damage of any nature whatsoever, whether direct or indirect, or for costs (including legal fees) and
expenses arising out of the publication, use of, or reliance upon, this IEC Publication or any other IEC
Publications.
8) Attention is drawn to the Normative references cited in this publication. Use of the referenced publications is
indispensable for the correct application of this publication.
9) Attention is drawn to the possibility that some of the elements of this IEC Publication may be the subject of
patent rights. IEC shall not be held responsible for identifying any or all such patent rights.
International Standard IEC 61010-2-101 has been prepared by IEC technical committee 66:
Safety of measuring, control and laboratory equipment.
It has the status of a group safety publication, as specified in IEC Guide 104.
This standard has been prepared in close collaboration with Working Group CENELEC
BTTF 88.1.
This second edition cancels and replaces the first edition published in 2002. It constitutes a
technical revision and includes the following significant changes from the first edition, as well
as numerous other changes:
• excluded IEC 61010-2-081 (general laboratory equipment) from the scope. This separates
IEC 61010-2-081 and IEC 61010-2-101 equipment;
– 4 – IEC 61010-2-101:2015 © IEC 2015
• updated Biohazard and Lot symbols in Table 1 in Clause 5;
• added requirement for within expiration consumables and authorized representative
details in Instructions for Use to Clause 5;
• added requirement for gas or liquid markings and ratings to Clause 5;
• added requirement to include OPERATOR instructions to deal with consumable or sample
spills, jams or breakage inside equipment, disposal of hazardous waste, personal
protection, RISK reduction procedures relating to flammable liquids, burns from surfaces,
and loading and unloading of sample and reagents in Instructions for Use to Clause 5;
• added requirement for manufacturer to provide instructions on equipment transport,
storage and removal from use to Clause 5;
• added normative reference ISO 18113-5 for instructions for use of self-test IVD medical
equipment in Clause 5;
• added requirement for OPERATOR maintenance instructions to Clause 7;
• added requirements for sample zones and loading zones to Clause 7;
• excluded equipment whose size and weight make unintentional movement unlikely from
drop test in Clause 8;
• added requirement for biohazard marking to Clause 13;
• added requirement for interlock systems containing electric/electronic or programmable
components to Clause 15;
• added informative reference to Usability standard IEC 62366 to Clause 16;
• replaced Clause 17 with requirements of ISO 14971 for RISK assessment.
• Annex BB Instructions for use for self-testing IVD Medical Equipment deleted and a
reference given to ISO 18113-5 in Clause 5.
The text of this standard is based on the following documents:
FDIS Report on voting
66/545/FDIS 66/560/RVD
Full information on the voting for the approval of this standard can be found in the report on
voting indicated in the above table.
This publication has been drafted in accordance with the ISO/IEC Directives, Part 2.
A list of all parts of the IEC 61010 series, under the general title: Safety requirements for
electrical equipment for measurement, control, and laboratory use, may be found on the IEC
website.
This Part 2-101 is intended to be used in conjunction with IEC 61010-1. It was established on
the basis of the third edition (2010).
This Part 2-101 supplements or modifies the corresponding clauses in IEC 61010-1 so as to
convert that publication into the IEC standard: Safety requirements for in vitro diagnostic
(IVD) medical equipment.
Where a particular subclause of Part 1 is not mentioned in this Part 2, that subclause applies
as far as is reasonable. Where this part states “addition”, “modification”, “replacement”, or
“deletion” the relevant requirement, test specification or note in Part 1 should be adapted
accordingly.
In this standard:
1) the following print types are used:
– requirements: in roman type;
– NOTES: in smaller roman type;
– conformity and test: in italic type;
– terms used throughout this standard which have been defined in clause 3: SMALL
ROMAN CAPITALS;
2) subclauses, figures, tables and notes which are additional to those in part 1 are numbered
starting from 101. Additional annexes are lettered starting from AA.
The committee has decided that the contents of this publication will remain unchanged until
the stability date indicated on the IEC web site under "http://webstore.iec.ch" in the data
related to the specific publication. At this date, the publication will be
• reconfirmed,
• withdrawn,
• replaced by a revised edition, or
• amended.
– 6 – IEC 61010-2-101:2015 © IEC 2015
SAFETY REQUIREMENTS FOR ELECTRICAL EQUIPMENT
FOR MEASUREMENT, CONTROL AND LABORATORY USE –
Part 2-101: Particular requirements for
in vitro diagnostic (IVD) medical equipment
1 Scope and object
This clause of Part 1 is applicable except as follows:
1.1.1 Equipment included in scope
Replacement:
Replace the text by the following:
This part of IEC 61010 applies to equipment intended for in vitro diagnostic (IVD) medical
purposes, including self-test IVD medical purposes.
IVD medical equipment, whether used alone or in combination, is intended by the
manufacturer to be used in vitro for the examination of specimens, including blood and tissue
samples, derived from the human body, solely or principally for the purpose of providing
information concerning one or more of the following:
• a physiological or pathological state; or
• a congenital abnormality;
• the determination of safety and compatibility with potential recipients;
• the monitoring of therapeutic measures.
Self-test IVD medical equipment is intended by the manufacturer for use by lay persons in a
home environment.
NOTE If all or part of the equipment falls within the scope of one or more other part 2 standards of IEC 61010 as
well as within the scope of this standard, considerations have to be given to those other part 2 standards.
1.1.2 Equipment excluded from scope
Addition:
Add the following item:
aa) Equipment in the scope of IEC 61010-2-081 unless they are specifically intended by
their manufacturer to be used for in vitro diagnostic examination.
1.2 Object
1.2.1 Aspects included in scope
Addition:
Add two items:
aa) biohazards;
bb) hazardous chemical substances.
1.2.2 Aspects excluded from scope
Addition:
Add the following item and note:
aa) the handling or manipulation outside the equipment of material under analysis.
NOTE Requirements covering these subjects are the responsibility of committees preparing relevant standards.
2 Normative references
This clause of Part 1 is applicable except as follows:
Addition:
Add the following references:
ISO 14971, Medical devices – Application of risk management to medical devices
ISO 18113-5, In vitro diagnostic medical devices – Information supplied by the manufacturer
(labelling) – In vitro diagnostic instruments for selftesting
ISO 13857, Safety of machinery – Safety distances to prevent hazard zones being reached by
upper and lower limbs
3 Terms and definitions
This clause of Part 1 is applicable except as follows:
3.1 Equipment and states of equipment
Addition:
Add the following terms and definitions:
3.1.101
SAMPLE ZONE
area where OPERATOR access is typically unintended; the inside of this zone presents
mechanical HAZARDS and a more likely probability of biohazardous human skin puncture
3.1.102
LOADING ZONE
area of automated equipment where an OPERATOR handles sample or reagent material.
3.5.12 RESPONSIBLE BODY
Addition:
Add the following note:
NOTE 1 This is not the European Community responsible authority.
4 Tests
This clause of Part 1 is applicable:
– 8 – IEC 61010-2-101:2015 © IEC 2015
5 Marking and documentation
This clause of Part 1 is applicable except as follows:
5.1.1 General
Replacement:
Replace the third paragraph by the following:
Letter symbols for quantities and units shall be in accordance with IEC 60027. Internationally
recognized symbols, including those of Table 1, shall be used as far as possible. If other
additional symbols are required, it shall not be possible to confuse them with the
internationally recognized symbols. There are no colour requirements for symbols. Graphic
symbols shall be explained in the documentation.
Table 1 – Symbols
Addition:
Add the following symbols to Table 1:
Number Symbol Publication Description
Background colour
– optional;
Symbol colour
101 ISO 7000- 0659 (2004-01) Biological RISKS
– optional;
Outline / outline colour
– optional;
102 ISO 7000- 2492 (2004-01) Batch code
5.1.2 Identification
Replacement:
Replace the text by the following:
Equipment shall, as a minimum, be marked with the following information:
a) manufacturer’s name or trade mark, and the address. The address shall include at least
the city and country;
NOTE 1 National regulation may require more details on the address than required in a).
b) model number, name, or other means of identifying the equipment;
The following additional information shall be marked on the equipment or packaging or in the
instructions for use:
1) the serial number, for example SN XXXX or alternatively the batch code, preceded by
‘LOT’, using symbol 102 of Table 1;
2) the following information:
i) a clear indication that the equipment is IVD medical equipment;
ii) if applicable, a clear indication that the equipment is self-test IVD medical
equipment;
iii) if a potential RISK is posed, the identification of detachable components by
manufacturer and part identification, and where appropriate the batch code, etc.
3) instructions for use shall require that the OPERATOR only use consumables that are
within their expiration date. Where this is required by regulation, the name and address
of the authorized representative of the manufacturer.
NOTE 2 For example, in the European Union this is the natural or legal person as established within
the European Community.
5.1.5 TERMINALS, connections and operating devices
Addition:
Add the following subclause:
5.1.5.101 Gas and liquid connections
If necessary for safety, the equipment shall be clearly marked near to the connector on the
equipment with;
a) a means of identifying the gas or liquid to be used. Where no internationally recognized
symbol (including chemical formulae) exists, the equipment shall be marked with
symbol 14 of Table 1;
b) the maximum permitted pressure, or alternatively symbol 14 of Table 1 (see 5.4.3).
Conformity is checked by inspection.
Addition:
Add the following subclause:
5.1.101 Transport and storage
Packaging of equipment shall be labelled to indicate any special conditions for transport or
storage (see 5.4.102).
Conformity is checked by inspection.
5.2 Warning markings
Replacement:
Replace the first paragraph by the following:
Warning Markings specified in 5.1.5.1, 5.1.5.2 c), 5.1.5.2 d), 5.1.5.101, 6.1.2 b), 7.3.2 b) 3),
7.4, 10.1, 13.2.2 and 13.101 shall meet the following requirements:
5.3 Durability of markings
Replacement:
Replace the first paragraph by the following paragraph:
Markings required by 5.1.2 to 5.2 shall remain clear and legible under conditions of NORMAL
USE, and resist the effects of temperature and rubbing, and of solvent and reagents likely to
– 10 – IEC 61010-2-101:2015 © IEC 2015
be encountered in NORMAL USE, including cleaning and decontaminating agents specified by
the manufacturer.
Addition:
Add the following paragraph after the second paragraph:
If a solvent or reagent specified for use with the equipment could affect the durability of a
particular marking, that marking is also rubbed for 30 s with the most frequently used and/or
aggressive solvent or reagent to which the equipment is likely to be exposed in NORMAL USE
A representative sample of groups of solvents or reagents likely to have a similar effect can
optional be used.
5.4.1 General
Deletion:
Delete the note 2 in the second paragraph.
5.4.3 Equipment installation
Replacement:
Replace subclause 5.4.3 by the following:
5.4.3 Equipment transportation, installation and assembly instructions
Documentation for the RESPONSIBLE BODY shall include the following if applicable:
a) instructions for transportation after delivery to the RESPONSIBLE BODY;
b) floor loading requirements;
NOTE Mass and dimensions are sufficient information for floor loading.
c) individual mass of heavy units;
d) location and mounting instructions, including the space required for ventilation, and for
safe and efficient OPERATOR maintenance;
e) assembly instructions;
f) instructions for protective earthing;
g) the sound data required by 12.5.1;
h) instructions relating to the handling, containment and exhaust of hazardous substances,
including any requirements for preventing back-syphonage;
i) any drainage systems required where a HAZARD could occur from the discharge of
biological and chemical substances and hot fluids;
j) details of protective measures relating to hazardous radiation (see clause 12);
k) connections to the supply;
l) for PERMANENTLY CONNECTED EQUIPMENT only:
1) MAINS supply requirements and details of connections, including the RATED temperature
of the cable required at maximum RATED ambient temperature;
2) requirements for any external switch or circuit-breaker (see 6.11.2.1) and external
overcurrent protection devices (see 9.6.1) and a recommendation that the switch or
circuit-breaker be near the equipment if this is necessary for safety;
m) requirements for special services (for example air, cooling liquid) including pressure limits.
Conformity is checked by inspection of the documentation.
5.4.4 Equipment operation
Replacement:
Replace the first paragraph text by the following:
Instructions for use shall include if applicable:
a) details of operating controls and their use in all operating modes; with any sequence of
operation;
NOTE 1 IEC 60073 gives guidance on colours and symbols of operating controls.
b) an instruction not to position the equipment so that it is difficult to operate the
disconnecting device (see 6.11);
c) instructions for interconnections to accessories and other equipment, including details of
suitable accessories, detachable parts and any special consumable materials;
d) limits for intermittent operation;
e) an explanation of symbols used on the equipment and, where HAZARDS are involved, the
reason for using a symbol in each particular case;
f) instructions for any actions to be taken by an OPERATOR to deal with a HAZARD resulting
from equipment spills, lock-ups, container breakage and similar malfunctions
g) instructions and recommendations for cleaning and decontamination, with materials
recommended (see 11.2);
h) instructions for the disposal of hazardous waste;
i) if NORMAL USE involves the handling of hazardous chemical substances, instructions on
correct use and any need for training or personal protection measures;
j) Appropriate instruction to use personal protective equipment (e.g. gloves, gowns) where
there could be contact with the skin when handling potentially infectious substances or
surfaces (such as human samples or reagents);
k) Appropriate instructions and requirements for protection of the mouth, nose or eyes shall
be given where the equipment could emit hazardous aerosol vapours in NORMAL USE;
l) Appropriate instructions and requirements for protective devices, such as protective
glasses shall be given where potentially hazardous visible or invisible radiation could be
emitted;
m) detailed instructions about RISK reduction procedures relating to flammable liquids (see
9.5 c));
n) details of methods of reducing the RISKS of burns from surfaces permitted to exceed the
temperature limits of 10.1.
o) Appropriate warnings to reduce RISK during loading and unloading of samples and
reagents (see 7.3.102)
p) Instructions for the RESPONSIBLE BODY to ensure that all retaining hardware (e.g screws,
fasteners) are in place on removable PROTECTIVE BARRIERS, and the removable PROTECTIVE
BARRIERS are in place on the instrument during normal operation.
q) A statement that, if a TOOL is required to remove a fixed PROTECTIVE BARRIER and/or
ENCLOSURE guarding a SAMPLE ZONE, access to that tool should be controlled by the
RESPONSIBLE BODY.
r) A statement listing the tools to be controlled by the RESPONSIBLE BODY.
NOTE 2 Information on decontaminants their use, dilution and potential application is contained in the Laboratory
Biosafety Manual, published by the World Health Organization and the Biosafety in Microbiological and Biomedical
Laboratories, published by Centers for Disease Control and Prevention and National Institutes of Health.
Washington. There are also national guidelines that cover these areas.
– 12 – IEC 61010-2-101:2015 © IEC 2015
NOTE 3 Cleaning and decontamination may be necessary as a safeguard when equipment and their accessories
are maintained, repaired or transferred. Preferably manufacturers provide a format for the RESPONSIBLE BODY to
certify to those maintaining, repairing or transferring equipment that such a treatment has been carried out.
Conformity is checked by inspection of the documentation.
Addition:
Add the following subclauses:
5.4.4.101 Instructions for use, self-test IVD medical equipment
Instructions for use of self-test IVD medical equipment shall comply with ISO 18113-5.
5.4.101 Removal of equipment from use for repair or disposal
Instructions shall be provided for the RESPONSIBLE BODY for eliminating or reducing HAZARDS
involved in removal from use, transportation or disposal, or appropriate contact information
shall be provided in the documentation.
NOTE Regional or international requirements can apply.
Conformity is checked by inspection of the documentation.
5.4.102 Transport and storage
The manufacturer shall specify the conditions for transport and storage of the equipment. The
documentation shall contain a specification of the permissible environmental conditions for
transport and storage. Essential information shall be repeated on the outside of the package
using appropriate symbols (see 5.1.101).
When the manufacturer assumes responsibility for delivery and installation the above is not
required in the documentation.
Compliance is checked by inspection.
6 Protection against electric shock
This clause of part 1 is applicable.
7 Protection against mechanical HAZARDS
This clause of part 1 is applicable, except as follows:
7.3.1 General
Replacement:
Replace the second sentence as follows:
The conditions specified in 7.3.4, 7.3.5, and 7.3.101 are considered to represent a tolerable
level.
Replace the conformity statement as follows:
Conformity is checked as specified in 7.3.2, 7.3.3, 7.3.4, 7.3.5, 7.3.101, and Clause 17 as
applicable.
7.3.2 Exceptions
Replacement:
Replace item b) 3) text by the following:
There are warning markings prohibiting access by untrained OPERATORS. Markings shall be
placed within the area requiring maintenance where they can alert the OPERATOR to the
HAZARD. As an alternative, symbol 14 of Table 1 can be used, with the warnings included in
the documentation.
Addition:
Add the following item to to the list:
b) 4) There are OPERATOR maintenance instructions that specify safe maintenance
procedures.
7.3.3 RISK assessment for mechanical HAZARDS to body parts
Replacement:
Replace text by the following:
If equipment is specified by the manufacturer for continuous loading of sample and reagent
materials and associated HAZARDS in the SAMPLE ZONE are solely caused by the sample and/or
reagent probes 7.3.101 applies specifically for the SAMPLE ZONE. Subclause 7.3.101 does not
apply to self-testing and point of care equipment.
RISKS shall be reduced to a tolerable level by at least the applicable minimum protective
measure of Table 12, taking into account the severity, probability of exposure and possibility
of avoiding the HAZARD.
Conformity is checked by evaluation of the RISK assessment documentation to ensure that the
RISKS have been eliminated or that only TOLERABLE RISKS remain.
Table 12 – Protective measures against mechanical HAZARDS to body parts
Replacement:
Replace the text of item B by the following text:
Moderate measures; emergency switches, PROTECTIVE BARRIERS or covers removable only
with a TOOL, distances (see ISO 13857), or separations (see ISO 13854 or EN 349).
Addition:
Add the following subclause:
7.3.101 SAMPLE ZONE
Equipment with a SAMPLE ZONE shall comply with the requirements of one or more of the
following:
aa) PROTECTIVE BARRIER or
bb) all following measures apply:
1) The minimum maintained gap between LOADING ZONE and SAMPLE ZONE is 120 mm.
– 14 – IEC 61010-2-101:2015 © IEC 2015
2) Unintentional contact between OPERATOR and sample/reagent pipettor is unlikely.
3) The area between LOADING ZONE and SAMPLE ZONE is marked with symbol 14 and
symbol 101 of table 1 (see 5.4.4 o)), or if not visible by the OPERATOR the marking shall
be located visible and close to the area.
8 Resistance to mechanical stresses
This clause of part 1 is applicable except as follows:
8.1 General
Replacement:
Replace the text of item 3) by the following:
3) except for FIXED EQUIPMENT, for equipment with a mass over 100 kg, or for equipment
whose size and weight make unintentional movement unlikely and which is not moved in
NORMAL USE, the appropriate test of 8.3. The equipment is not operated during the tests.
Addition:
Add the following subclause:
8.101 Transport and storage
When delivered in the manufacturer’s packaging, equipment shall not cause a HAZARD during
NORMAL USE after transport or storage in the conditions specified by the manufacturer
(see 5.1.101 and 5.4.101).
If the manufacturer assumes responsibility for delivery and installation, the above requirement
is met without inspection of test records.
Conformity is checked by inspection of records of transport tests performed by the
manufacturer.
NOTE Guidance on tests is given in ASTM D4169, and in the publications of the International Safe Transport
Association (ISTA).
9 Protection against the spread of fire
This clause of Part 1 is applicable.
10 Equipment temperature limits and resistance to heat
This clause of Part 1 is applicable.
11 Protection against HAZARDS from fluids
This clause of Part 1 is applicable:
12 Protection against radiation, including laser sources, and against sonic and
ultrasonic pressure
This clause of Part 1 is applicable.
13 Protection against liberated gases and substances, explosion and implosion
This clause of Part 1 is applicable except as follows:
Addition:
Add the following subclause:
13.101 Biohazardous substances
Equipment that can be potentially infectious due to the samples or reagents used shall be
prominently marked with symbol 101 of Table 1. At minimum, a biohazard symbol shall be
near the sampling area and visible in NORMAL USE.
Biohazard symbols shall be near biohazardous areas accessed during OPERATOR maintenance
visible only during this maintenance.
Symbol 101 of Table 1 shall be marked on containers or bags for biohazardous waste material
which can be removed from the equipment during NORMAL USE, and near any biohazardous
drain connection.
Equipment that can be hazardous due to the use of hazardous substances shall be marked
with the appropriate international symbol, or (if none is available) symbol 14 of Table 1.
14 Components and subassemblies
This clause of Part 1 is applicable except as follows:
14.3 Over-temperature protection devices
Addition:
Add the following paragraph after the second paragraph:
Over-temperature protection devices in self-test IVD medical equipment shall not be self-
resetting.
15 Protection by interlocks
This clause of Part 1 is applicable except as follows.
15.1 General
Addition:
Add the following text after the first sentence:
As an alternative method, for interlock systems containing electric/electronic or programmable
components (E/E/P components) the reliability and design requirements can be determined by
applying e.g. IEC 62061 (SIL) or ISO 13849 (PL) or other solutions providing equivalent
functional safety.
16 HAZARDS resulting from application
This clause of Part 1 is applicable except as follows:
– 16 – IEC 61010-2-101:2015 © IEC 2015
16.2 Ergonomic aspects
Replacement:
Replace the note by the following note:
NOTE RISK assessment procedures for ergonomics can be found in IEC 62366, EN 894-2, EN 894-3, ISO 9241,
SEMI S8 and other documents. Not all of the requirements in these documents will be applicable to equipment
within the scope of this standard.
17 RISK assessment
This clause of Part 1 is replaced as follows:
Replacement:
RISK assessment shall be carried out and documented using the requirements of ISO 14971
for HAZARDS not addressed in this standard and Part 1.
Conformity is checked by evaluation of the RISK assessment documentation to assure that the
RISKS have been eliminated or that only TOLERABLE RISKS remain.
Annexes
The annexes of Part 1 are applicable except as follows:
Annex L
(informative)
Index of defined terms
Addition:
Add the following defined terms to the list:
SAMPLE ZONE . 3.1.101
LOADING ZONE . 3.1.102
– 18 – IEC 61010-2-101:2015 © IEC 2015
Bibliography
Addition:
Add the following references:
EN 980:2008, Graphical symbols for use in the labelling of medical devices
ISO 15223-1 Medical devices – Symbols to be used with medical device labels, labelling and
information to be supplied – Part 1: General requirements
ASTM D4169, Standard practice for performance testing for shipping containers
Laboratory Biosafety Manual, World Health Organization
Biosafety in Microbiological and Biomedical Laboratories, published by Centers for Disease
Control and Prevention and National Institutes of Health
Publications of the International Safe Transport Association (ISTA).
_____________
– 20 – IEC 61010-2-101:2015 © IEC 2015
SOMMAIRE
AVANT-PROPOS . 21
1 Domaine d’application et objet . 24
2 Références normatives . 25
3 Termes et définitions . 25
4 Essais . 26
5 Marquage et documentation . 26
6 Protection contre les chocs électriques . 31
7 Protection contre les DANGERS mécaniques . 31
8 Résistance aux contraintes mécaniques . 32
9 Protection contre la propagation du feu . 33
10 Limites de température de l’appareil et résistance à la chaleur . 33
11 Protection contre les DANGERS provenant des fluides . 33
12 Protection contre les radiations, y compris les sources laser, et contre la pression
acoustique et ultrasonique . 33
13 Protection contre les émissions de gaz et de substances et contre les explosions
et les implosions . 33
14 Composants et sous-ensembles . 34
15 Protection par systèmes de verrouillage . 34
16 DANGERS résultant de l’application . 34
17 Evaluation du RISQUE . 35
Annexes . 35
Annexe L (informative) Index des termes définis . 36
Bibliographie . 37
Tableau 1 – Symboles . 26
COMMISSION ÉLECTROTECHNIQUE INTERNATIONALE
____________
RÈGLES DE SÉCURITÉ POUR APPAREILS ÉLECTRIQUES
DE MESURAGE, DE RÉGULATION ET DE LABORATOIRE –
Partie 2-101: Exigences particulières pour les appareils
médicaux de diagnostic in vitro (DIV)
AVANT-PROPOS
1) La Commission Electrotechnique Internationale (IEC) est une organisation mondiale de normalisation
composée de l'ensemble des comités électrotechniques nationaux (Comités nationaux de l’IEC). L’IEC a pour
objet de favor
...



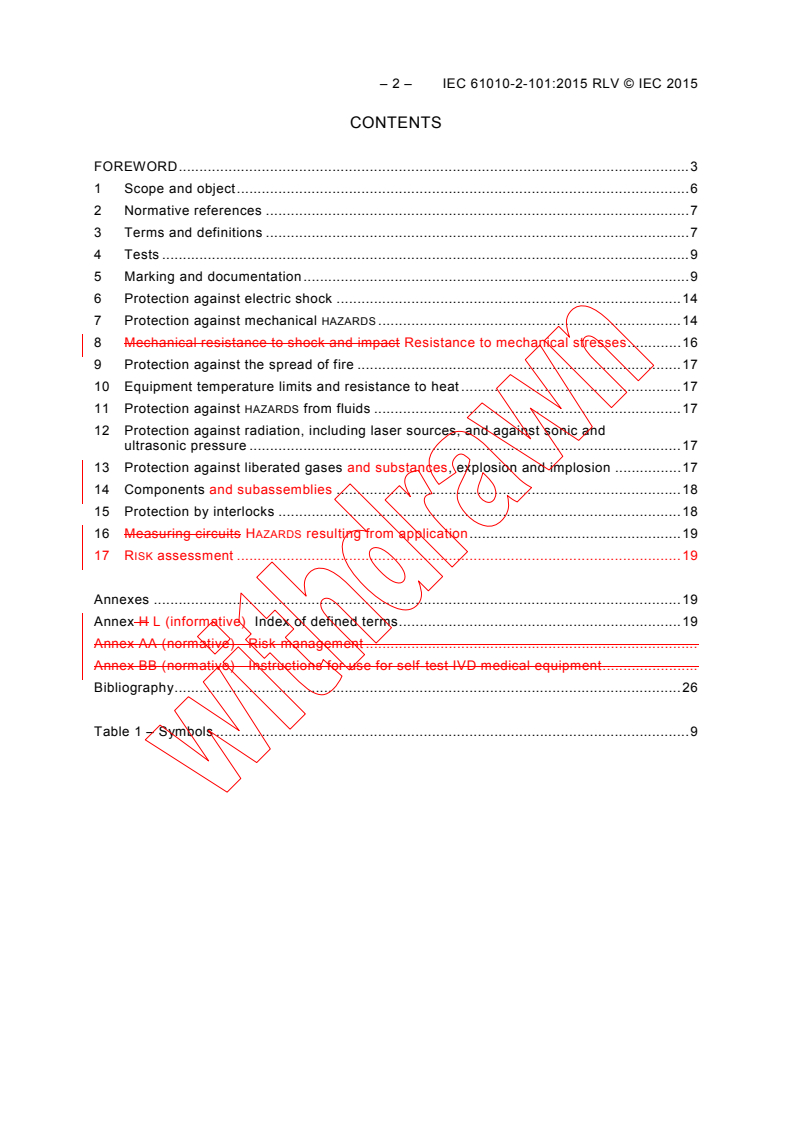




Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.
Loading comments...