ASTM D6667-01
(Test Method)Standard Test Method for Determination of Total Volatile Sulfur in Gaseous Hydrocarbons and Liquefied Petroleum Gases by Ultraviolet Fluorescence
Standard Test Method for Determination of Total Volatile Sulfur in Gaseous Hydrocarbons and Liquefied Petroleum Gases by Ultraviolet Fluorescence
SCOPE
1.1 This test method covers the determination of total volatile sulfur in gaseous hydrocarbons and liquefied petroleum (LP) gases. It is applicable to analysis of natural, processed, and final product materials containing sulfur in the range of 1 to 100 mg/kg (Note 1).
Note 1--An estimate of pooled limit of quantification (PLOQ), information regarding sample stability and other general information derived from the inter-laboratory study on precision can be referenced in ASTM research report RR: D02-1506.
1.2 This test method may not detect sulfur compounds that do not vaporize under the conditions of the test.
1.3 This test method is applicable for total volatile sulfur determination in LP gases containing less than 0.35 % (mass/mass) halogen(s).
1.4 The values stated in SI units are to be regarded as standard.
1.5 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.See paragraphs 3.1, 6.3, and Section 7 for specific safety statements.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
An American National Standard
Designation: D 6667 – 01
Standard Test Method for
Determination of Total Volatile Sulfur in Gaseous
Hydrocarbons and Liquefied Petroleum Gases by Ultraviolet
Fluorescence
This standard is issued under the fixed designation D 6667; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope D 5287 Practice for the Automatic Sampling of Gaseous
Fuels
1.1 This test method covers the determination of total
D 6299 Practice for Applying Statistical Quality Assurance
volatile sulfur in gaseous hydrocarbons and liquefied petro-
Techniques to Evaluate Analytical Measurement System
leum (LP) gases. It is applicable to analysis of natural,
Performance
processed, and final product materials containing sulfur in the
F 307 PracticeforSamplingPressurizedGasforGasAnaly-
range of 1 to 100 mg/kg (Note 1).
sis
NOTE 1—An estimate of pooled limit of quantification (PLOQ), infor- 8
2.2 Gas Processor Association (GPA) Standards:
mation regarding sample stability and other general information derived
GPA2166 Obtaining Natural Gas Samples forAnalysis by
from the inter-laboratory study on precision can be referenced in ASTM
Gas Chromatography
research report RR: D02-1506.
GPA 2174 Obtaining Liquid Hydrocarbon Samples for
1.2 This test method may not detect sulfur compounds that
Analysis by Gas Chromatography
do not vaporize under the conditions of the test.
1.3 This test method is applicable for total volatile sulfur
3. Summary of Test Method
determination in LP gases containing less than 0.35 % (mass/
3.1 Aheatedsamplevalveisusedtoinjectgaseoussamples.
mass) halogen(s).
Liquefied petroleum gas (LPG) samples are injected by a
1.4 The values stated in SI units are to be regarded as
sample valve connected to a heated expansion chamber. The
standard.
gaseous sample then enters a high temperature combustion
1.5 This standard does not purport to address all of the
tube where sulfur is oxidized to sulfur dioxide (SO)inan
safety concerns, if any, associated with its use. It is the
oxygen rich atmosphere. Water produced during the sample
responsibility of the user of this standard to establish appro-
combustion is removed and the sample combustion gases are
priate safety and health practices and determine the applica-
next exposed to ultraviolet (UV) light. The SO absorbs the
bility of regulatory limitations prior to use. See paragraphs 3.1,
energy from the UV light and is converted to an excited sulfur
6.3, and Section 7 for specific safety statements. *
dioxide (SO ). Fluorescence emitted from the excited SO as
2 2
*
it returns to a stable state SO is detected by a photomultiplier
2. Referenced Documents
tube,theresultingsignalisameasureofthesulfurcontainedin
2.1 ASTM Standards:
the sample. (Warning—Exposure to excessive quantities of
D 1070 Test Methods for Relative Density (Specific Grav-
ultraviolet light is injurious to health. The operator shall avoid
ity) of Gaseous Fuels
exposing their person, especially their eyes, not only to direct
D 1265 Practice for Sampling Liquefied Petroleum (LP)
UV light but also to secondary or scattered radiation that is
Gases (Manual Method)
present.)
D 3700 PracticeforContainingHydrocarbonFluidSamples
Using a Floating Piston Cylinder
4. Significance and Use
4.1 The sulfur content of LPG, used for fuel purposes,
contributes to SOx emissions and can lead to corrosion in
This test method is under the jurisdiction of ASTM Committee D02 on
engine and exhaust systems. Some process catalysts used in
Petroleum Products and Lubricantsand is the direct responsibility of Subcommittee
petroleum and chemical refining can be poisoned by sulfur
D02.03on Elemental Analysis.
Current edition approved May 10, 2001. Published July 2001.
Available from ASTM Headquarters. Request RR:D02–1506.
3 6
Annual Book of ASTM Standards, Vol 05.06. Annual Book of ASTM Standards, Vol 05.03.
4 7
Annual Book of ASTM Standards, Vol 05.01. Annual Book of ASTM Standards, Vol 15.03.
5 8
Annual Book of ASTM Standards, Vol 05.02. Available from Gas Processors Assoc., 6526 East 60th, Tulsa, OK 74145.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
D6667–01
FIG. 1 Example of a Typical Direct Inject Quartz Pyrolysis Tube
bearing materials in the feed stocks. This test method can be 5.7 Strip Chart Recorder, equivalent electronic data logger,
used to determine sulfur in process feeds, to measure sulfur in integrator or, recorder (optional).
finished products, and can also be used for compliance deter-
minations when acceptable to a regulatory authority. 6. Reagents
6.1 Purity of Reagents—Reagent grade chemicals shall be
5. Apparatus
used in tests. Unless otherwise indicated, it is intended that all
5.1 Furnace—An electric furnace held at a temperature
reagents shall conform to the specifications of the Committee
(1075 6 25°C) sufficient to pyrolyze the entire sample and
on Analytical Reagents of the American Chemical Society,
oxidize sulfur to SO .
2 where such specifications are available. Other grades may be
5.2 Combustion Tube—A quartz combustion tube con-
used, provided it is first ascertained that the reagent is of
structed to allow the direct injection of the sample into the
sufficiently high purity to permit its use without lessening the
heated oxidation zone of the furnace. The combustion tube
accuracy of the determination.
shall have side arms for the introduction of oxygen and carrier
6.2 Inert Gas—Argon or helium only, high purity grade
gas. The oxidation section shall be large enough (see Fig. 1) to
(that is, chromatography or zero grade), 99.998 % min purity,
ensure complete combustion of the sample (see 11.3). Fig. 1
moisture 5 mg/kg max. (Warning—Argon or helium may be a
depicts a typical combustion tube. Other configurations are
compressed gas under high pressure (7.1)).
acceptable when precision is not degraded.
6.3 Oxygen—High purity (that is chromatography or zero
5.3 Flow Control—The apparatus shall be equipped with
grade), 99.75 % min purity, moisture 5 mg/kg max, dried over
flow controllers capable of maintaining a constant supply of
molecular sieves. (Warning—Oxygen vigorously accelerates
oxygen and carrier gas at the specified rates.
combustion and may be compressed gas under high pressure
5.4 Drier Tube—The apparatus shall be equipped with a
(7.1)).
mechanism for the removal of water vapor formed during
6.4 Calibration Standards—Certified calibration standards
sample combustion. This can be accomplished with a mem-
from commercial sources or calibration gases prepared using
brane drying tube, or a permeation dryer that utilizes a
certified permeation tube devices are required. Table 1 lists the
selective capillary action for water removal.
sulfur source material and diluent matrices used during the
5.5 UV Fluorescence Detector—A quantitative detector ca-
inter-laboratory study (Notes 2 and 3).
pable of measuring light emitted from the fluorescence of
NOTE 2—Other sulfur sources and diluent materials may be used if
sulfur dioxide by UV light.
precision and accuracy are not degraded.
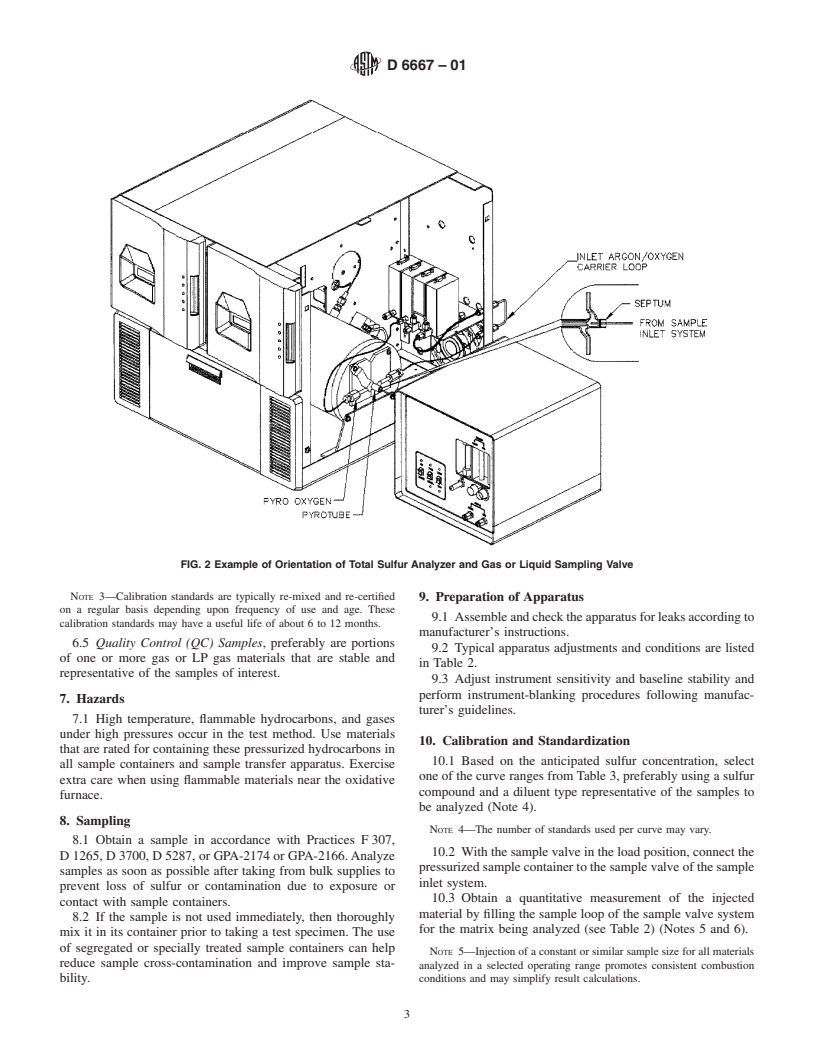
5.6 Sample Inlet System—The system provides a heated
gas-sampling valve, or a LP gas-sampling valve, or both, with
a heated expansion chamber, connected to the inlet of the
oxidation area, Fig. 2. The system is swept by an inert carrier
Reagent Chemicals, American Chemical Society Specifications, American
Chemical Society, Washington, DC. For suggestions on the testing of reagents not
gas and shall be capable of allowing the quantitative delivery
listed by the American Chemical Society, see Analar Standards for Laboratory
of the material to be analyzed into the oxidation zone at a
Chemicals, BDH Ltd., Poole, Dorset, U.K., and the United States Pharmacopeia
controlled and repeatable rate of approximately 30 mL/min.
and National Formulary, U.S. Pharmacopeial Convention, Inc. (USPC), Rockville,
Fig. 3 provides an example. MD.
D6667–01
FIG. 2 Example of Orientation of Total Sulfur Analyzer and Gas or Liquid Sampling Valve
NOTE 3—Calibration standards are typically re-mixed and re-certified
9. Preparation of Apparatus
on a regular basis depending upon frequency of use and age. These
9.1 Assembleandchecktheapparatusforleaksaccordingto
calibration standards may have a useful life of about 6 to 12 months.
manufacturer’s instructions.
6.5 Quality Control (QC) Samples, preferably are portions
9.2 Typical apparatus adjustments and conditions are listed
of one or more gas or LP gas materials that are stable and
in Table 2.
representative of the samples of interest.
9.3 Adjust instrument sensitivity and baseline stability and
perform instrument-blanking procedures following manufac-
7. Hazards
turer’s guidelines.
7.1 High temperature, flammable hydrocarbons, and gases
under high pressures occur in the test method. Use materials
10. Calibration and Standardization
that are rated for containing these pressurized hydrocarbons in
10.1 Based on the anticipated sulfur concentration, select
all sample containers and sample transfer apparatus. Exercise
one of the curve ranges from Table 3, preferably using a sulfur
extra care when using flammable materials near the oxidative
compound and a diluent type representative of the samples to
furnace.
be analyzed (Note 4).
8. Sampling
NOTE 4—The number of standards used per curve may vary.
8.1 Obtain a sample in accordance with Practices F 307,
10.2 With the sample valve in the load position, connect the
D 1265, D 3700, D 5287, or GPA-2174 or GPA-2166.Analyze
pressurized sample container to the sample valve of the sample
samples as soon as possible after taking from bulk supplies to
inlet system.
prevent loss of sulfur or contamination due to exposure or
10.3 Obtain a quantitative measurement of the injected
contact with sample containers.
material by filling the sample loop of the sample valve system
8.2 If the sample is not used immediately, then thoroughly
for the matrix being analyzed (see Table 2) (Notes 5 and 6).
mix it in its container prior to taking a test specimen. The use
of segregated or specially treated sample containers can help
NOTE 5—Injection of a constant or similar sample size for all materials
reduce sample cross-contamination and improve sample sta-
analyzed in a selected operating range promotes consistent combustion
bility. conditions and may simplify result calculations.
D6667–01
FIG. 3 Sample Inlet System Flow Path
TABLE 1 Typical Standard Materials TABLE 4 Repeatability (r) and Reproducibility (R)
Sulfur Source Diluent Concentration
rR
(mg/kg S)
Dimethyl sulfide n, butane
iso-butane 1 0.1 0.3
propylene 5 0.6 1.6
propane 10 1.2 3.1
25 2.9 7.8
50 5.8 15.6
100 11.5 31.3
TABLE 2 Typical Operating Conditions
Sample inlet system temperature 85 6 20°C
Sample injection system carrier gas 25–30 mL/min
10.5 Calibrate the instrument using one of the following
Furnace temperature 1075 6 25°C
techniques.
Furnace oxygen flow meter setting 375–450 mL/min
Inlet oxygen flow meter setting 10–30 mL/min 10.5.1 Multi-point Calibration:
Inlet carrier flow meter setting 130–160 mL/min
10.5.1.1 When the apparatus features an internal self-
Gas sample size 10–20 mL
calibration routine, analyze the calibration standards and blank
LPG sample size 15 µL
three times using the procedures described in 10.2-10.4.
10.5.1.2 Calibrate the analyzer according to the manufac-
TABLE 3 Sulfur Standards turer’s instructions to yield sulfur concentration (see Section
13).Thiscurveistypicallylinearandsystemperformanceshall
Curve I Curve II
Sulfur mg/kg Sulfur mg/kg
be checked at least once per day, each day of use. (Note 7).
Blank Blank
NOTE 7—Other calibration curve techniques may be used when accu-
5.00 10.00
racy and precision are not degraded. The frequency of calibration may be
10.00 50.00
determined by the use of quality control charts or other quality assurance/
100.00
quality control techniques.
10.5.2 One-point Calibration:
NOTE 6—An automatic sample transfer and injection device may be
10.5.2.1 Utilize a calibration standard (6.4) with a sulfur
used.
content close to that of the samples to be analyzed (625 %
10.3.1 Flush the sample loop with sufficient calibrant to max.).
assure that the material to be injected is representative. 10.5.2.2 Follow the instrument manufacturer’s instructions
10.3.2 For LPG samples, if bubbles are present in the to establish an instrument zero (instrument blank) by conduct-
viewable portion of the liquid column, flush the sample loop to ing an analysis run without injection of the calibration stan-
introduce a new liquid-full sample portion. dard.
10.4 Start the analyzer and inject the calibration material 10.5.2.3 Performmeasurementsofthecalibrationstandarda
according to the manufacturer’s instructions. minimum of three times.
D6667–01
10.5.2.4 Calculate a calibration factor K, in counts per
Dc = density of calibration standard at measurement tem-
nanogram of sulfur (counts/ng S) as described in 12.2.
perature, g/mL,
Vc = volume of calibration standard injected, µL,
11. Procedure Scg = sulfur content of calibration standard, mL/kg, and
Scv = sulfur content of calibration standard, mg/L.
11.1 Obtain a test specimen using the procedure described
12.2.1 Calculate the average of the calibration factor (K)
in Section 8. Typically the sulfur concentration in the test
and check that the standard deviation is within the tolerance
specimen is less than the concentration of the highest standard
accepted.This calibration factor shall be established every day.
and greater than the concentration of the lowest standard used
12.2.2 Calculate the sulfur content, S, of the sample, in
in the calibration.
mg/kg, using the following equation:
11.2 Measure the response for the test specimen using one
A
of the procedures described in 10.2-10.4.
S 5 (5)
M 3 K 3 Fg
11.3 Inspect the combustion tube and other flow path
components to verify complete oxidation of the test specimen.
or
11.3.1 Reduce the rate of injection or the sample size, or
A
both, of the specimen into the furnace when coke or sooting is
S 5 (6)
V 3 K 3 Fv
observed.
11.4 Cleaning and Re-calibration—Clean any coked or
where:
sooted parts according to the manufacturer’s instructions.After K = calibration factor, in counts per nanogram of sulfur,
any cleaning or adjustment, assemble and check the apparatus and
M = mass of test s
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.