ASTM D3505-96(2006)
(Test Method)Standard Test Method for Density or Relative Density of Pure Liquid Chemicals
Standard Test Method for Density or Relative Density of Pure Liquid Chemicals
SIGNIFICANCE AND USE
This test method is suitable for setting specification, for use as an internal quality control tool, and for use in development or research work on industrial aromatic hydrocarbons and related materials. In addition to the pure liquid chemicals for which expansion functions are known, it may also be used for liquids for which temperature expansion data are not available, or for impure liquid chemicals if certain limitations are observed. Information derived from this test can be used to describe the relationship between weight and volume.
SCOPE
1.1 This test method describes a simplified procedure for the measurement of density or relative density of pure liquid chemicals for which accurate temperature expansion functions are known. It is restricted to liquids having vapor pressures not exceeding 600 mm Hg (0.8 atm) at the equilibration temperature, and having viscosities not exceeding 15 cSt at 20C (60F).
1.2 Means are provided for reporting results in the following units:
Density g/cm3 at 20C
Density g/ml at 20C
Relative density 20C/4C
Relative density 60F/60F (15.56C/15.56C)
Commercial density, lb (in air)/U.S. gal at 60F
Commercial density, lb (in air)/U.K. gal at 60F. Note 1
This test method is based on the old definition of 1 L = 1.000028 dm3 (1 mL = 1.000028 cm3). In 1964 the General Conference on Weights and Measures withdrew this definition of the litre and declared that the word "litre" was a special name for the cubic decimetre, thus making 1 mL = 1 cm3 exactly.
An alternative method for determining relative density of pure liquid chemicals is Test Method D 4052.
1.3 The following applies to all specified limits in this test method: for purposes of determining conformance with this test method, an observed value or a calculated value shall be rounded off "to the nearest unit" in the last right-hand digit used in expressing the specification limit, in accordance with the rounding-off method of Practice E 29.
This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use. Specific hazard statements are given in .
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation:D3505–96 (Reapproved 2006)
Standard Test Method for
Density or Relative Density of Pure Liquid Chemicals
This standard is issued under the fixed designation D3505; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
This standard has been approved for use by agencies of the Department of Defense.
1. Scope 2. Referenced Documents
1.1 Thistestmethoddescribesasimplifiedprocedureforthe 2.1 ASTM Standards:
measurement of density or relative density of pure liquid D1193 Specification for Reagent Water
chemicals for which accurate temperature expansion functions D1555 Test Method for Calculation of Volume and Weight
areknown.Itisrestrictedtoliquidshavingvaporpressuresnot of Industrial Aromatic Hydrocarbons and Cyclohexane
exceeding 600 mm Hg (0.8 atm) at the equilibration tempera- D3437 Practice for Sampling and Handling Liquid Cyclic
ture, and having viscosities not exceeding 15 cSt at 20°C Products
(60°F). D4052 Test Method for Density, Relative Density, andAPI
1.2 Means are provided for reporting results in the follow- Gravity of Liquids by Digital Density Meter
ing units: E1 Specification for ASTM Liquid-in-Glass Thermometers
Density g/cm at 20°C E12 Terminology Relating to Density and Specific Gravity
Density g/ml at 20°C of Solids, Liquids, and Gases
Relative density 20°C/4°C E29 Practice for Using Significant Digits in Test Data to
Relative density 60°F/60°F (15.56°C/15.56°C) Determine Conformance with Specifications
Commercial density, lb (in air)/U.S. gal at 60°F 2.2 Other Document:
Commercial density, lb (in air)/U.K. gal at 60°F. OSHA Regulations, 29 CFR paragraphs 1910.1000 and
1910.1200
NOTE 1—This test method is based on the old definition of 1
3 3
L=1.000028 dm (1 mL=1.000028 cm ). In 1964 the General Confer-
3. Terminology
ence on Weights and Measures withdrew this definition of the litre and
3.1 Definitions:
declared that the word “litre” was a special name for the cubic decimetre,
thus making 1 mL=1 cm exactly.
3.1.1 density, n—the mass of material per unit volume at a
NOTE 2—An alternative method for determining relative density of
given temperature called the “reference temperature.” Weight
pure liquid chemicals is Test Method D4052.
correctedtoastandardaccelerationofgravityandcorrectedfor
1.3 The following applies to all specified limits in this test the buoyant effect of air is used to measure mass.This method
method: for purposes of determining conformance with this specifiestheuseofabeambalancetodetermineweightsothat
test method, an observed value or a calculated value shall be no correction for variation in acceleration of gravity is neces-
rounded off “to the nearest unit” in the last right-hand digit sary.When a torsion or spring balance is used, such correction
used in expressing the specification limit, in accordance with must be applied.
the rounding-off method of Practice E29. 3.1.2 relative density, n—the ratio of the density of the
1.4 This standard does not purport to address all of the material at reference temperature “t” to the density of pure
safety concerns, if any, associated with its use. It is the water, in consistent units, at reference temperature t.Itis
responsibility of the user of this standard to establish appro- common practice to use reference temperature t equal to t .
1 2
priate safety and health practices and determine the applica-
bility of regulatory limitations prior to use. Specific hazard
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
statements are given in 7.1.
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Standards volume information, refer to the standard’s Document Summary page on
This test method is under the jurisdiction of ASTM Committee D16 on the ASTM website.
Aromatic Hydrocarbons and Related Chemicals and is the direct responsibility of Withdrawn. The last approved version of this historical standard is referenced
Subcommittee D16.04 on Instrumental Analysis. on www.astm.org.
Current edition approved Jan. 1, 2006. Published January 2006. Originally AvailablefromU.S.GovernmentPrintingOfficeSuperintendentofDocuments,
approved in 1976. Last previous edition approved in 2000 as D3505–96(2000). 732 N. Capitol St., NW, Mail Stop: SDE, Washington, DC 20401, http://
DOI: 10.1520/D3505-96R06. www.access.gpo.gov.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
D3505–96 (2006)
3.1.2.1 Since the mass of water at 4°C is very close to 1 nient temperature between 10 and 30°C (50 and 86°F). The
g/mL or 1 g/cm , it is common practice to set the reference equilibriumtemperatureismeasuredtothenearest0.02°C.The
temperature t for water at 4°C. When this is done and the weight is determined using a beam balance. The density,
densityofthematerialisgiveningramspermillilitre,orgrams relative density, or commercial density at the desired reference
per cubic centimetre, the value of density is very nearly temperature is then calculated from the sample weight, a
identicaltothevalueforrelativedensity.Thus,densityat20°C calibrationfactorproportionaltoanequalvolumeofwater,and
in g/cm or g/mL, is nearly identical with relative density a multiplier which corrects for the buoyancy of air and the
20°C/4°C. change in volume of the pycnometer and the sample due to
deviation from the chosen reference temperature.
3.1.3 commercial density, n—weight per unit volume with-
outcorrectingforthebuoyanteffectofairandislimitedinthis 4.2 For liquids not listed in Table 1, the sample is equili-
document to pounds (in air) per U.S. gallon at 60°F, or pounds brated at the desired reference temperature, usually 20°C or
in air per U.K. gallon at 60°F. This is the density most 60°F (15.56°C), the density, relative density, or commercial
commonly used in commercial transactions in the petroleum densityisthencalculatedfromthesampleweight,acalibration
and coal chemicals industry in the United States and Canada. factor proportional to an equal volume of water and a term
which corrects for the buoyancy of air. In the case of volatile
3.2 The definitions included in Terminology E12 are appli-
liquidssuchaspentane,thetimebetweenreadingofvolumeat
cable to this test method.
the equilibrium temperature and weighing must not be pro-
longed, otherwise weight loss through evaporation may result
4. Summary of Test Method
in errors.
NOTE 3—SeeAppendix X1 for details on the method and derivation of
formulas.
4.1 For materials listed in Table 1 the sample is drawn into
For a more complete discussion on the use of this design pycnometer, see
a weighed and calibrated bicapillary pycnometer. The filler
Lipken, Davidson, Harvey and Kurtz, Industrial Engineering Chemistry, Analytical
pycnometer is allowed to come to equilibrium at any conve- Edition; Vol 16, 1944, p. 55.
D3505–96 (2006)
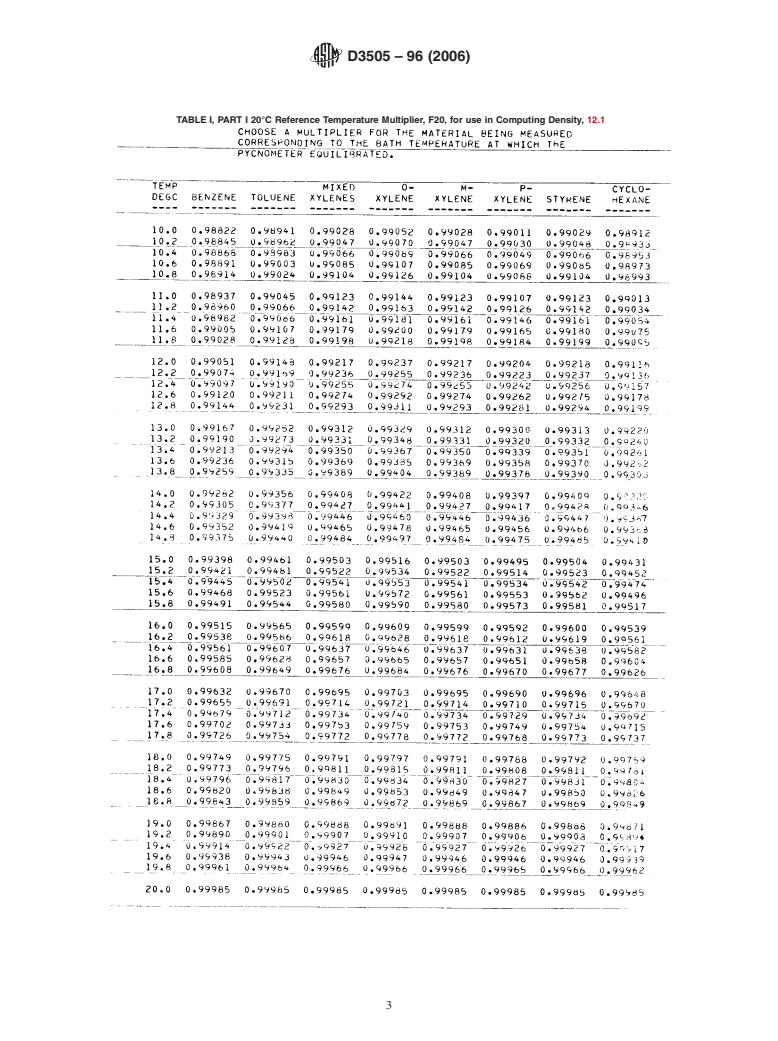
TABLEI, PART I 20°C Reference Temperature Multiplier, F20, for use in Computing Density, 12.1
D3505–96 (2006)
TABLEI, PART I Continued
D3505–96 (2006)
TABLEI, PART II 60°F Reference Temperature Multiplier, F60, for use in Computing Density, 12.1
D3505–96 (2006)
TABLEI, PART II Continued
D3505–96 (2006)
5. Significance and Use
5.1 This test method is suitable for setting specification, for
use as an internal quality control tool, and for use in develop-
mentorresearchworkonindustrialaromatichydrocarbonsand
related materials. In addition to the pure liquid chemicals for
which expansion functions are known, it may also be used for
liquidsforwhichtemperatureexpansiondataarenotavailable,
or for impure liquid chemicals if certain limitations are
observed. Information derived from this test can be used to
describe the relationship between weight and volume.
6. Apparatus
6.1 Pycnometer, 9 to 10-mL capacity, conforming to the
dimensions given in Fig. 1, constructed of borosilicate glass,
and having a total weight not exceeding 30 g.
6.2 Bath, having a depth of at least 300 mm, capable of
being maintained constant to 60.02°C at any convenient
temperature between 10°C (50°F) and 30°C (86°F). Provide a
support for the pycnometer (see Fig. 2) constructed of any
suitable noncorrosive metal.
NOTE 1—All dimensions are in inches.
FIG. 2 Pycnometer Holder
8. Sampling
8.1 SamplethematerialinaccordancewithPracticeD3437.
9. Preparation of Apparatus
9.1 Acid Cleaning, for use when the pycnometer is to be
calibrated or when liquid fails to drain cleanly from the walls
of the pycnometer or its capillary. Thoroughly clean with hot
chromic acid solution and rinse well with reagent water
NOTE 1—The graduation lines shall extend around the entire circum-
conforming to Type III of Specification D1193. Other suitable
ferenceofthepycnometerattheintegralnumbers0,1,2cm,etc.,halfway
around at the half divisions 0.5, 1.5, etc., and shorter lines for the
cleaning procedures may be used. Dry at 105 to 110°C for at
intermediate subdivisions.
least 1 h, preferably with a slow current of filtered air passing
FIG. 1 Pycnometer
through the pycnometer.
9.2 Solvent Cleaning,forusebetweendeterminations.Rinse
NOTE 4—If the laboratory air temperature does not vary more than
with toluene and then with anhydrous acetone, drying with a
0.02°C during temperature equilibration a special bath is not needed.
filtered stream of dry air.
6.3 Bath Thermometer, An ASTM Precision Thermometer,
10. Calibration of Apparatus
having a range from−8 to+32°C and conforming to the
requirements for Thermometer 63C as prescribed in Specifi- 10.1 UsingtheproceduredescribedinSection11,determine
cation E1.
the weight of freshly boiled reagent water conforming to Type
III of Specification D1193 held by the pycnometer with the
7. Hazards
water level at each of three different scale points on the
7.1 Consult current OSHA regulations, supplier’s Material graduated arms. Two of these water levels must be at opposite
SafetyDataSheets,andlocalregulations,forallmaterialsused ends of the scale. Make all weighings on the same day, using
in this test method. the same balance and weights.
D3505–96 (2006)
A
TABLE 2 Density of Water , g/ml
t,° C 0.0 0.1 0.2 0.3 0.4 0.5 0.56 0.6 0.7 0.8 0.9
15 0.999 13 11 10 08 07 05 04 04 02 00 *99
16 0.998 97 96 94 92 91 89 87 86 84 82
17 80 79 77 75 73 72 70 68 66 64
18 62 61 59 57 55 53 51 49 47 45
19 43 42 40 38 36 34 32 30 27 25
20 23 21 19 17 15 13 11 09 07 04
21 02 00 *98 *96 *93 *91 *89 *87 *85 *82
22 0.997 80 78 75 73 71 69 66 64 62 59
23 57 54 52 50 47 45 42 40 38 35
24 33 30 28 25 23 20 18 15 13 10
25 08 05 02 00 *97 *95 *92 *89 *87 *84
26 0.996 81 79 76 73 71 68 65 63 60 57
27 54 52 49 46 43 41 38 35 32 29
28 26 24 21 18 15 12 09 06 03 00
29 0.995 98 95 92 89 86 83 80 77 74 72
30 68 65 62 59 56 53 50 46 43 40
A
Abstracted from Tilton and Taylor, U.S. National Bureau of Standards Research Paper 971, NBS Journal of Research Vol 18, 1917, p. 213. This paper is a statistical
analysis of the data of Chappuis, Travaux Et Memoires du Bureau International de Poid et Mesures, Vol 13, 1907, p. D39.
p
10.2 Calculate the volume, V , at each scale point tested siphonwhentheliquidlevelinthebulbarmofthepycnometer
T
by means of the following equation; carry all calculations in 6
reaches the lowest graduation mark.
non-zero digits and round to 4 decimal places:
11.3 Thoroughly dry the wet tip. Wipe the body of the
p w w
pycnometer with a chemically clean, lint-free cloth slightly
Pycnometercapacity, V ,mL 5 A 3 ~W /d ! 1 B~T 2 t! (1)
T t
damp with water (Note 4) and weigh the filled pycnometer to
where:
the nearest 0.1 mg.
A = airbuoyancycoefficient,aconstantforthetempera-
ture range involved=1.001064 NOTE 5—In atmospheres below 60% relative humidity, drying the
p
V = volume of pycnometer at reference temperature, T pycnometer by rubbing with a dry cotton cloth will induce static charges
T
w
equivalent to a loss of about 1 mg or more in the weight of the
W = weightofwaterinair,containedinthepycnometer,
pycnometer. This charge may not be completely dissipated in less than ⁄2
g
w
, and can be detected by touching the pycnometer to the wire hook in the
d = density of water at t (see Table 2)
t
balance and then drawing it away slowly. If the pycnometer exhibits an
t = test temperature, °C
attraction for the wire hook, it may be considered to have a static charge.
T = reference temperature, 20°C or 15.56°C, and
B = volumetric coefficient of expansion of 9.5 mL of a
11.4 Place the pycnometer in the holder in a constant-
−5
borosilicateglasspycnometer,9.26276 310 mL/
temperature bath held at any convenient temperature 10 and
°C.
30°C within 60.02°C; for materials not listed in Table 1, hold
10.3 Prepare a calibration curve by plotting apparent vol-
the bath exactly at the desired reference temperature, usually
ume, V , that is, the sum of the scale readings on the two arms
A
15.56°C or 20°C. When the liquid level has reached tempera-
of the pycnometer against the corresponding calculated vol-
tureequilibrium(usuallyinabout10min)andwhilestillinthe
p
ume, V . If a straight line cannot be drawn through the three
T
bath, read the scale to the nearest 0.2 small division at the
points, discard the data and determine three additional points
liquid level in each arm.
sothatastraightcalibrationlinecanbedrawnsuchthatnodata
point lies more than 0.0002-mL units from the line. If neither
12. Calculation
set of data meets the condition, the diameters of the graduated
capillaryarmsarenotsufficientlyuniform,andthepycnometer 12.1 Table 1 Materials—Compute the density or relative
should be discarded.
density, or both, by means of the following equations:
10.4 From the curve obtained, prepare a table of apparent
s
W
volume, V , (sum of scale readings of both arms), as apparent
Density,g/mLat60°F 5 3 F 10.00121 (2)
A
p 60
p V
volume against corresponding calculated volumes, V ,in
T
s
increments of 0.0001 mL. Label this table with the reference W
Density,g/mLat20°C 5 3 F 10.00121 (3)
p 20
temperature to which it applies.
V
s
W
11. Procedure
Density,g/cm at20°C 5 F 10.00121 0.99997 (4)
F p 20 G
V
11.1 Weigh the clean, dry pycnometer to 0.1 mg and record
s
W
the weight.
Relativedensity60/60°F 5 3 F 10.00121 1.00096 (5)
F p 60 G
11.2 With the sample at approxim
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.