ASTM D6667-04(2010)
(Test Method)Standard Test Method for Determination of Total Volatile Sulfur in Gaseous Hydrocarbons and Liquefied Petroleum Gases by Ultraviolet Fluorescence
Standard Test Method for Determination of Total Volatile Sulfur in Gaseous Hydrocarbons and Liquefied Petroleum Gases by Ultraviolet Fluorescence
SIGNIFICANCE AND USE
The sulfur content of LPG, used for fuel purposes, contributes to SOx emissions and can lead to corrosion in engine and exhaust systems. Some process catalysts used in petroleum and chemical refining can be poisoned by sulfur bearing materials in the feed stocks. This test method can be used to determine sulfur in process feeds, to measure sulfur in finished products, and can also be used for compliance determinations when acceptable to a regulatory authority.
SCOPE
1.1 This test method covers the determination of total volatile sulfur in gaseous hydrocarbons and liquefied petroleum (LP) gases. It is applicable to analysis of natural, processed, and final product materials containing sulfur in the range of 1 to 100 mg/kg (Note 1).
Note 1—An estimate of pooled limit of quantification (PLOQ), information regarding sample stability and other general information derived from the inter-laboratory study on precision can be referenced in the ASTM research report.
1.2 This test method may not detect sulfur compounds that do not vaporize under the conditions of the test.
1.3 This test method is applicable for total volatile sulfur determination in LP gases containing less than 0.35 % (mass/mass) halogen(s).
1.4 The values stated in SI units are to be regarded as standard.
1.5 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use. See 3.1 and Sections 6 and 7 for specific warning statements.
General Information
Relations
Buy Standard
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: D6667 – 04 (Reapproved 2010)
Standard Test Method for
Determination of Total Volatile Sulfur in Gaseous
Hydrocarbons and Liquefied Petroleum Gases by Ultraviolet
Fluorescence
This standard is issued under the fixed designation D6667; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope D3700 Practice for Obtaining LPG Samples Using a Float-
ing Piston Cylinder
1.1 This test method covers the determination of total
D5287 Practice for Automatic Sampling of Gaseous Fuels
volatile sulfur in gaseous hydrocarbons and liquefied petro-
D6299 Practice for Applying Statistical Quality Assurance
leum (LP) gases. It is applicable to analysis of natural,
and Control Charting Techniques to Evaluate Analytical
processed, and final product materials containing sulfur in the
Measurement System Performance
range of 1 to 100 mg/kg (Note 1).
F307 Practice for Sampling Pressurized Gas for GasAnaly-
NOTE 1—An estimate of pooled limit of quantification (PLOQ), infor-
sis
mation regarding sample stability and other general information derived
2.2 Gas Processor Association (GPA) Standards:
from the inter-laboratory study on precision can be referenced in the
GPA2166 Obtaining Natural Gas Samples forAnalysis by
ASTM research report.
Gas Chromatography
1.2 This test method may not detect sulfur compounds that
GPA 2174 Obtaining Liquid Hydrocarbon Samples for
do not vaporize under the conditions of the test.
Analysis by Gas Chromatography
1.3 This test method is applicable for total volatile sulfur
determination in LP gases containing less than 0.35 % (mass/
3. Summary of Test Method
mass) halogen(s).
3.1 Aheatedsamplevalveisusedtoinjectgaseoussamples.
1.4 The values stated in SI units are to be regarded as
Liquefied petroleum gas (LPG) samples are injected by a
standard.
sample valve connected to a heated expansion chamber. The
1.5 This standard does not purport to address all of the
gaseous sample then enters a high temperature combustion
safety concerns, if any, associated with its use. It is the
tube where sulfur is oxidized to sulfur dioxide (SO)inan
responsibility of the user of this standard to establish appro-
oxygen rich atmosphere. Water produced during the sample
priate safety and health practices and determine the applica-
combustion is removed and the sample combustion gases are
bility of regulatory limitations prior to use. See 3.1 and
next exposed to ultraviolet (UV) light. The SO absorbs the
Sections 6 and 7 for specific warning statements.
energy from the UV light and is converted to an excited sulfur
*
dioxide (SO ). Fluorescence emitted from the excited SO as
2 2
2. Referenced Documents
*
it returns to a stable state SO is detected by a photomultiplier
2.1 ASTM Standards:
tube,theresultingsignalisameasureofthesulfurcontainedin
D1070 Test Methods for Relative Density of Gaseous Fuels
the sample. (Warning—Exposure to excessive quantities of
D1265 Practice for Sampling Liquefied Petroleum (LP)
ultraviolet light is injurious to health. The operator shall avoid
Gases, Manual Method
exposing their person, especially their eyes, not only to direct
UV light but also to secondary or scattered radiation that is
present.)
This test method is under the jurisdiction of ASTM Committee D02 on
Petroleum Products and Lubricants and is the direct responsibility of Subcommittee
4. Significance and Use
D02.03 on Elemental Analysis.
Current edition approved May 1, 2010. Published May 2010. Originally
4.1 The sulfur content of LPG, used for fuel purposes,
approved in 2001. Last previous edition approved in 2004 as D6667–04. DOI:
contributes to SOx emissions and can lead to corrosion in
10.1520/D6667-10.
engine and exhaust systems. Some process catalysts used in
Supporting data have been filed at ASTM International Headquarters and may
be obtained by requesting Research Report RR:D02-1506.
petroleum and chemical refining can be poisoned by sulfur
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Standards volume information, refer to the standard’s Document Summary page on Available from Gas ProcessorsAssociation (GPA), 6526 E. 60th St.,Tulsa, OK
the ASTM website. 74145.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
D6667 – 04 (2010)
FIG. 1 Example of a Typical Direct Inject Quartz Pyrolysis Tube
bearing materials in the feed stocks. This test method can be 5.7 Strip Chart Recorder, equivalent electronic data logger,
used to determine sulfur in process feeds, to measure sulfur in integrator or, recorder (optional).
finished products, and can also be used for compliance deter-
minations when acceptable to a regulatory authority.
6. Reagents
6.1 Purity of Reagents—Reagent grade chemicals shall be
5. Apparatus
used in tests. Unless otherwise indicated, it is intended that all
5.1 Furnace—An electric furnace held at a temperature
reagents shall conform to the specifications of the Committee
(1075 6 25°C) sufficient to pyrolyze the entire sample and 5
on Analytical Reagents of the American Chemical Society,
oxidize sulfur to SO .
where such specifications are available. Other grades may be
5.2 Combustion Tube—A quartz combustion tube con-
used, provided it is first ascertained that the reagent is of
structed to allow the direct injection of the sample into the
sufficiently high purity to permit its use without lessening the
heated oxidation zone of the furnace. The combustion tube
accuracy of the determination.
shall have side arms for the introduction of oxygen and carrier
6.2 Inert Gas—Argon or helium only, high purity grade
gas. The oxidation section shall be large enough (see Fig. 1)to
(that is, chromatography or zero grade), 99.998 % min purity,
ensure complete combustion of the sample (see 11.3). Fig. 1
moisture 5 mg/kg max. (Warning—Argon or helium may be a
depicts a typical combustion tube. Other configurations are
compressed gas under high pressure (7.1)).
acceptable when precision is not degraded.
6.3 Oxygen—High purity (that is chromatography or zero
5.3 Flow Control—The apparatus shall be equipped with
grade), 99.75 % min purity, moisture 5 mg/kg max, dried over
flow controllers capable of maintaining a constant supply of
molecular sieves. (Warning—Oxygen vigorously accelerates
oxygen and carrier gas at the specified rates.
combustion and may be compressed gas under high pressure
5.4 Drier Tube—The apparatus shall be equipped with a
(7.1)).
mechanism for the removal of water vapor formed during
6.4 Calibration Standards—Certified calibration standards
sample combustion. This can be accomplished with a mem-
from commercial sources or calibration gases prepared using
brane drying tube, or a permeation dryer that utilizes a
certified permeation tube devices are required. Table 1 lists the
selective capillary action for water removal.
sulfur source material and diluent matrices used during the
5.5 UV Fluorescence Detector—A quantitative detector ca-
inter-laboratory study (Notes 2 and 3).
pable of measuring light emitted from the fluorescence of
NOTE 2—Other sulfur sources and diluent materials may be used if
sulfur dioxide by UV light.
precision and accuracy are not degraded.
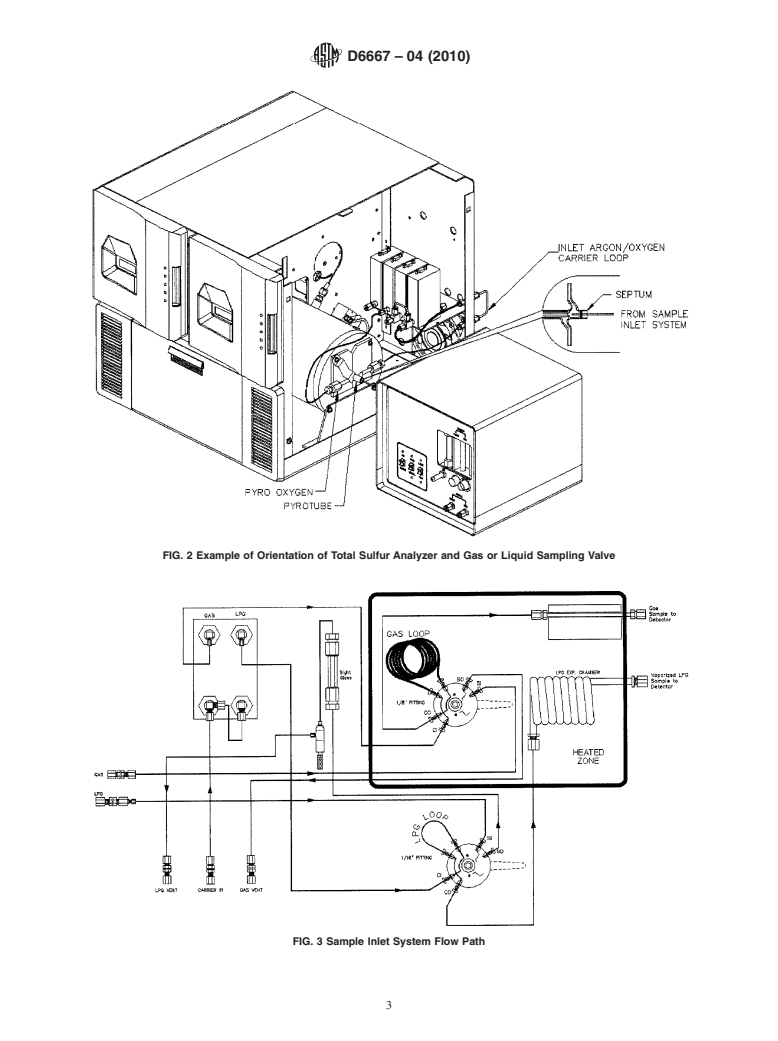
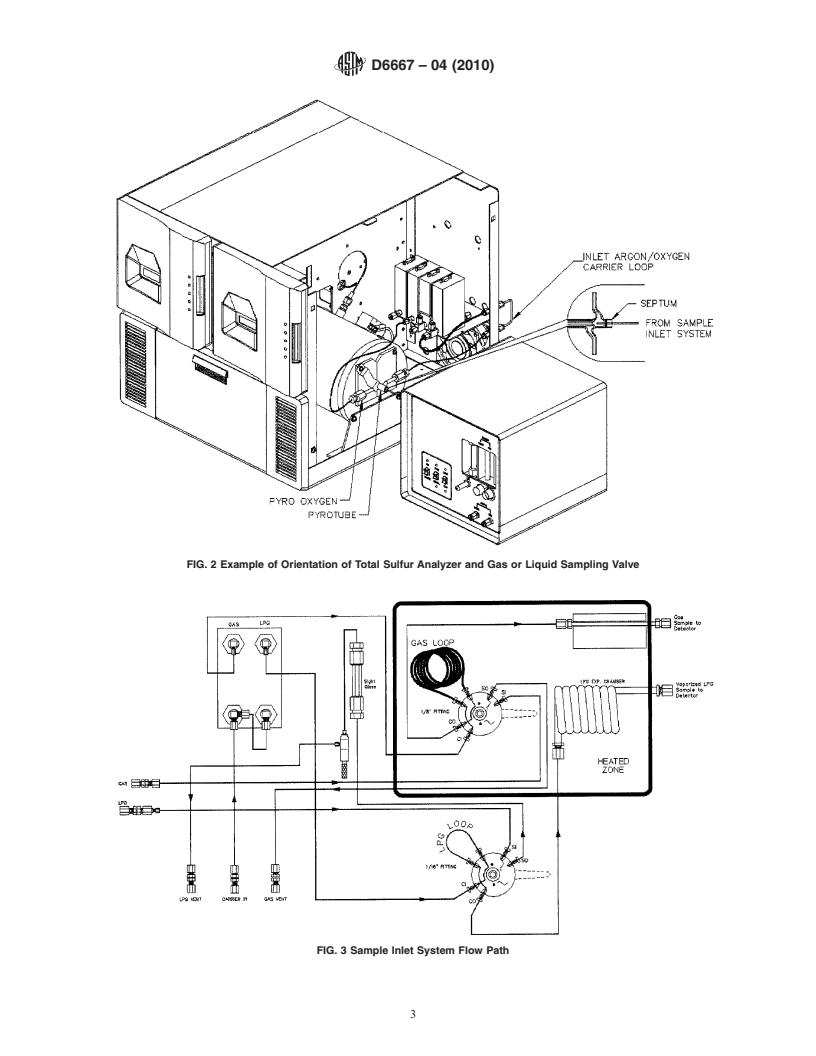
5.6 Sample Inlet System—The system provides a heated
gas-sampling valve, or a LP gas-sampling valve, or both, with
a heated expansion chamber, connected to the inlet of the
oxidation area, Fig. 2. The system is swept by an inert carrier
Reagent Chemicals, American Chemical Society Specifications, American
Chemical Society, Washington, DC. For suggestions on the testing of reagents not
gas and shall be capable of allowing the quantitative delivery
listed by the American Chemical Society, see Analar Standards for Laboratory
of the material to be analyzed into the oxidation zone at a
Chemicals, BDH Ltd., Poole, Dorset, U.K., and the United States Pharmacopeia
controlled and repeatable rate of approximately 30 mL/min.
and National Formulary, U.S. Pharmacopeial Convention, Inc. (USPC), Rockville,
Fig. 3 provides an example. MD.
D6667 – 04 (2010)
FIG. 2 Example of Orientation of Total Sulfur Analyzer and Gas or Liquid Sampling Valve
FIG. 3 Sample Inlet System Flow Path
D6667 – 04 (2010)
TABLE 1 Typical Standard Materials TABLE 3 Typical Sulfur Calibration Ranges and Standard
Concentrations
Sulfur Source Diluent
Curve I Curve II
Dimethyl sulfide n, butane
Sulfur mg/kg Sulfur mg/kg
iso-butane
propylene
Blank Blank
propane
5.00 10.00
10.00 50.00
100.00
NOTE 3—Calibration standards are typically re-mixed and re-certified
on a regular basis depending upon frequency of use and age. These
than those indicated may be used if desired. However, the
calibration standards may have a useful life of about 6 to 12 months.
method precision using narrower ranges than those indicated
6.5 Quality Control (QC) Samples, preferably are portions
has not been determined. Ensure the standards used for
of one or more gas or LP gas materials that are stable and
calibration bracket the concentrations of the samples being
representative of the samples of interest.
analyzed.
7. Hazards
NOTE 4—The number of standards used per curve may vary.
7.1 High temperature, flammable hydrocarbons, and gases
10.2 With the sample valve in the load position, connect the
under high pressures occur in the test method. Use materials
pressurized sample container to the sample valve of the sample
that are rated for containing these pressurized hydrocarbons in
inlet system.
all sample containers and sample transfer apparatus. Exercise
10.3 Obtain a quantitative measurement of the injected
extra care when using flammable materials near the oxidative
material by filling the sample loop of the sample valve system
furnace.
for the matrix being analyzed (see Table 2) (Notes 5 and 6).
NOTE 5—Injection of a constant or similar sample size for all materials
8. Sampling
analyzed in a selected operating range promotes consistent combustion
8.1 Obtain a sample in accordance with Practices F307,
conditions and may simplify result calculations.
D1265, D3700, D5287,or GPA 2174 or GPA 2166. Analyze
NOTE 6—An automatic sample transfer and injection device may be
samples as soon as possible after taking from bulk supplies to
used.
prevent loss of sulfur or contamination due to exposure or
10.3.1 Flush the sample loop with sufficient calibrant to
contact with sample containers.
assure that the material to be injected is representative.
8.2 If the sample is not used immediately, then thoroughly
10.3.2 For LPG samples, if bubbles are present in the
mix it in its container prior to taking a test specimen. The use
viewable portion of the liquid column, flush the sample loop to
of segregated or specially treated sample containers can help
introduce a new liquid-full sample portion.
reduce sample cross-contamination and improve sample sta-
10.4 Start the analyzer and inject the calibration material
bility.
according to the manufacturer’s instructions.
10.5 Calibrate the instrument using one of the following
9. Preparation of Apparatus
techniques.
9.1 Assembleandchecktheapparatusforleaksaccordingto
10.5.1 Multi-point Calibration:
manufacturer’s instructions.
10.5.1.1 When the apparatus features an internal self-
9.2 Typical apparatus adjustments and conditions are listed
calibration routine, analyze the calibration standards and blank
in Table 2.
three times using the procedures described in 10.2-10.4.
9.3 Adjust instrument sensitivity and baseline stability and
10.5.1.2 Calibrate the analyzer according to the manufac-
perform instrument-blanking procedures following manufac-
turer’s instructions to yield sulfur concentration (see Section
turer’s guidelines.
13).Thiscurveistypicallylinearandsystemperformanceshall
be checked at least once per day, each day of use. (Note 7).
10. Calibration and Standardization
NOTE 7—Other calibration curve techniques may be used when accu-
10.1 ConsultTable 3 and select a calibration range based on
racy and precision are not degraded. The frequency of calibration may be
the anticipated sulfur concentrations present in samples to be
determined by the use of quality control charts or other quality assurance/
analyzed, preferably using a sulfur compound and a diluent
quality control techniques.
type representative of the samples to be analyzed (Note 4).
10.5.2 One-point Calibration:
Table 3 is representative of typical ranges, but narrower ranges
TABLE 4 Repeatability (r) and Reproducibility (R)
TABLE 2 Typical Operating Conditions
Concentration
Sample inlet system temperature 85 6 20°C rR
(mg/kg S)
Sample injection system carrier gas 25–30 mL/min
Furnace temperature 1075 6 25°C 1 0.1 0.3
Furnace oxygen flow meter setting 375–450 mL/min 5 0.6 1.6
Inlet oxygen flow meter setting 10–30 mL/min 10 1.2 3.1
Inlet carrier flow meter setting 130–160 mL/min 25 2.9 7.8
Gas sample size 10–20 mL 50 5.8 15.6
LPG sample size 15 µL 100 11.5 31.3
D6667 – 04 (2010)
Ac
10.5.2.1 Utilize a calibration standard (6.4) with a sulfur
K 5 (3)
Vc 3 Scv
content close to that of the samples to be analyzed (625 %
max.).
where:
10.5.2.2 Follow the instrument manufacturer’s instructions
Ac = integrated detector response for calibration standard,
to establish an instrument zero (instrument blank) by conduct-
in counts, and
ing an analysis run without injection of the calibration stan-
Mc = mass of calibration standard injected, in milligrams,
dard.
either measured directly or calculated from measured
10.5.2.3 Performmeasurementsofthecalibrationstandarda
volume injected and density.
minimum of three times.
Mc 5 V 3 Dc (4)
10.5.2.4 Calculate a calibration factor K, in counts per
nanogram of sulfur (counts/ng S) as described in 12.2.
where:
Dc = density of calibration standard at measurement tem-
11. Procedure
perature, g/mL,
11.1 Obtain a test specimen using the procedure described
Vc = volume of calibration standard injected, µL,
in Section 8. Typically the sulfur concentration in the test
Scg = sulfur content of calibration standard, mL/kg, and
specimen is less than the concentration of the highest standard
Scv = sulfur content of calibration standard, mg/L.
and greater than the concentration of the lowest standard used
12.2.1 Calculate the average of the calibration factor (K)
in the calibration.
and check that the standard de
...
This document is not anASTM standard and is intended only to provide the user of anASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
An American National Standard Designation: D6667 – 04 (Reapproved 2010)
Designation:D 6667–04
Standard Test Method for
Determination of Total Volatile Sulfur in Gaseous
Hydrocarbons and Liquefied Petroleum Gases by Ultraviolet
Fluorescence
This standard is issued under the fixed designation D6667; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope *
1.1 This test method covers the determination of total volatile sulfur in gaseous hydrocarbons and liquefied petroleum (LP)
gases. It is applicable to analysis of natural, processed, and final product materials containing sulfur in the range of 1 to 100 mg/kg
(Note 1).
NOTE 1—An estimate of pooled limit of quantification (PLOQ), information regarding sample stability and other general information derived from the
inter-laboratory study on precision can be referenced in the ASTM research report.
1.2 This test method may not detect sulfur compounds that do not vaporize under the conditions of the test.
1.3 This test method is applicable for total volatile sulfur determination in LP gases containing less than 0.35 % (mass/mass)
halogen(s).
1.4 The values stated in SI units are to be regarded as standard.
1.5 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility
of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory
limitations prior to use. See 3.1 and Sections 6 and 7 for specific warning statements.
2. Referenced Documents
2.1 ASTM Standards:
D1070 Test Methods for Relative Density (Specific Gravity) of Gaseous Fuels
D1265 Practice for Sampling Liquefied Petroleum (LP) Gases (Manual Method) Practice for Sampling Liquefied Petroleum
(LP) Gases, Manual Method
D3700 Practice for Obtaining LPG Samples Using a Floating Piston Cylinder
D5287 Practice for the Automatic Sampling of Gaseous Fuels
D6299 Practice for Applying Statistical Quality Assurance and Control Charting Techniques to Evaluate Analytical
Measurement System Performance
F307 Practice for Sampling Pressurized Gas for Gas Analysis
2.2 Gas Processor Association (GPA) Standards:
GPA 2166 Obtaining Natural Gas Samples for Analysis by Gas Chromatography
GPA 2174 Obtaining Liquid Hydrocarbon Samples for Analysis by Gas Chromatography
3. Summary of Test Method
3.1 Aheated sample valve is used to inject gaseous samples. Liquefied petroleum gas (LPG) samples are injected by a sample
valve connected to a heated expansion chamber.The gaseous sample then enters a high temperature combustion tube where sulfur
is oxidized to sulfur dioxide (SO ) in an oxygen rich atmosphere. Water produced during the sample combustion is removed and
the sample combustion gases are next exposed to ultraviolet (UV) light. The SO absorbs the energy from the UV light and is
* *
converted to an excited sulfur dioxide (SO ). Fluorescence emitted from the excited SO as it returns to a stable state SO is
2 2 2
This test method is under the jurisdiction of ASTM Committee D02 on Petroleum Products and Lubricants and is the direct responsibility of Subcommittee D02.03 on
Elemental Analysis.
Current edition approved Nov. 1, 2004. Published November 2004. Originally approved in 2001. Last previous edition approved in 2001 as D 6667–01.
Current edition approved May 1, 2010. Published May 2010. Originally approved in 2001. Last previous edition approved in 2004 as D6667–04. DOI: 10.1520/D6667-10.
Supporting data have been filed at ASTM International Headquarters and may be obtained by requesting Research Report RR:D02–-1506.
For referencedASTM standards, visit theASTM website, www.astm.org, or contactASTM Customer Service at service@astm.org. For Annual Book of ASTM Standards
volume information, refer to the standard’s Document Summary page on the ASTM website.
Available from Gas Processors Association (GPA), 6526 E. 60th St., Tulsa, OK 74145.
*A Summary of Changes section appears at the end of this standard.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
D6667 – 04 (2010)
detected by a photomultiplier tube, the resulting signal is a measure of the sulfur contained in the sample. (Warning—Exposure
to excessive quantities of ultraviolet light is injurious to health. The operator shall avoid exposing their person, especially their
eyes, not only to direct UV light but also to secondary or scattered radiation that is present.)
4. Significance and Use
4.1 The sulfur content of LPG, used for fuel purposes, contributes to SOx emissions and can lead to corrosion in engine and
exhaust systems. Some process catalysts used in petroleum and chemical refining can be poisoned by sulfur bearing materials in
the feed stocks. This test method can be used to determine sulfur in process feeds, to measure sulfur in finished products, and can
also be used for compliance determinations when acceptable to a regulatory authority.
5. Apparatus
5.1 Furnace—An electric furnace held at a temperature (1075 6 25°C) sufficient to pyrolyze the entire sample and oxidize
sulfur to SO .
5.2 Combustion Tube—A quartz combustion tube constructed to allow the direct injection of the sample into the heated
oxidation zone of the furnace. The combustion tube shall have side arms for the introduction of oxygen and carrier gas. The
oxidationsectionshallbelargeenough(seeFig.1)toensurecompletecombustionofthesample(see11.3).Fig.1depictsatypical
combustion tube. Other configurations are acceptable when precision is not degraded.
5.3 Flow Control—The apparatus shall be equipped with flow controllers capable of maintaining a constant supply of oxygen
and carrier gas at the specified rates.
5.4 Drier Tube—The apparatus shall be equipped with a mechanism for the removal of water vapor formed during sample
combustion.Thiscanbeaccomplishedwithamembranedryingtube,orapermeationdryerthatutilizesaselectivecapillaryaction
for water removal.
5.5 UV Fluorescence Detector—A quantitative detector capable of measuring light emitted from the fluorescence of sulfur
dioxide by UV light.
5.6 Sample Inlet System—The system provides a heated gas-sampling valve, or a LPgas-sampling valve, or both, with a heated
expansion chamber, connected to the inlet of the oxidation area, Fig. 2. The system is swept by an inert carrier gas and shall be
capable of allowing the quantitative delivery of the material to be analyzed into the oxidation zone at a controlled and repeatable
rate of approximately 30 mL/min. Fig. 3 provides an example.
5.7 Strip Chart Recorder, equivalent electronic data logger, integrator or, recorder (optional).
6. Reagents
6.1 Purity of Reagents—Reagent grade chemicals shall be used in tests. Unless otherwise indicated, it is intended that all
FIG. 1 Example of a Typical Direct Inject Quartz Pyrolysis Tube
D6667 – 04 (2010)
FIG. 2 Example of Orientation of Total Sulfur Analyzer and Gas or Liquid Sampling Valve
FIG. 3 Sample Inlet System Flow Path
D6667 – 04 (2010)
reagents shall conform to the specifications of the Committee on Analytical Reagents of the American Chemical Society, where
such specifications are available. Other grades may be used, provided it is first ascertained that the reagent is of sufficiently high
purity to permit its use without lessening the accuracy of the determination.
6.2 Inert Gas—Argon or helium only, high purity grade (that is, chromatography or zero grade), 99.998 % min purity, moisture
5 mg/kg max. (Warning —Argon or helium may be a compressed gas under high pressure (7.1)).
6.3 Oxygen—High purity (that is chromatography or zero grade), 99.75 % min purity, moisture 5 mg/kg max, dried over
molecular sieves. (Warning—Oxygen vigorously accelerates combustion and may be compressed gas under high pressure (7.1)).
6.4 Calibration Standards—Certified calibration standards from commercial sources or calibration gases prepared using
certified permeation tube devices are required. Table 1 lists the sulfur source material and diluent matrices used during the
inter-laboratory study (Notes 2 and 3).
NOTE 2—Other sulfur sources and diluent materials may be used if precision and accuracy are not degraded.
NOTE 3—Calibration standards are typically re-mixed and re-certified on a regular basis depending upon frequency of use and age. These calibration
standards may have a useful life of about 6 to 12 months.
6.5 Quality Control (QC) Samples, preferably are portions of one or more gas or LP gas materials that are stable and
representative of the samples of interest.
7. Hazards
7.1 High temperature, flammable hydrocarbons, and gases under high pressures occur in the test method. Use materials that are
rated for containing these pressurized hydrocarbons in all sample containers and sample transfer apparatus. Exercise extra care
when using flammable materials near the oxidative furnace.
8. Sampling
8.1Obtain a sample in accordance with Practices F 307F 307, D 1265D 1265, D 3700D 3700, D 5287D 5287, or GPA-2174 or
GPA-2166
8.1 Obtain a sample in accordance with Practices F307, D1265, D3700, D5287, or GPA 2174 or GPA 2166. Analyze samples
as soon as possible after taking from bulk supplies to prevent loss of sulfur or contamination due to exposure or contact with
sample containers.
8.2 If the sample is not used immediately, then thoroughly mix it in its container prior to taking a test specimen. The use of
segregated or specially treated sample containers can help reduce sample cross-contamination and improve sample stability.
9. Preparation of Apparatus
9.1 Assemble and check the apparatus for leaks according to manufacturer’s instructions.
9.2 Typical apparatus adjustments and conditions are listed in Table 2.
9.3 Adjust instrument sensitivity and baseline stability and perform instrument-blanking procedures following manufacturer’s
guidelines.
10. Calibration and Standardization
10.1Consult
10.1 Consult Table 3 and select a calibration range based on the anticipated sulfur concentrations present in samples to be
analyzed, preferably using a sulfur compound and a diluent type representative of the samples to be analyzed (Note 4). Table 3
isrepresentativeoftypicalranges,butnarrowerrangesthanthoseindicatedmaybeusedifdesired.However,themethodprecision
using narrower ranges than those indicated has not been determined. Ensure the standards used for calibration bracket the
concentrations of the samples being analyzed.
NOTE 4—The number of standards used per curve may vary.
10.2 With the sample valve in the load position, connect the pressurized sample container to the sample valve of the sample
inlet system.
Reagent Chemicals, American Chemical Society Specifications, American Chemical Society, Washington, DC. For suggestions on the testing of reagents not listed by
the American Chemical Society, see Analar Standards for Laboratory Chemicals, BDH Ltd., Poole, Dorset, U.K., and the United States Pharmacopeia and National
Formulary, U.S. Pharmacopeial Convention, Inc. (USPC), Rockville, MD.
TABLE 1 Typical Standard Materials
Sulfur Source Diluent
Dimethyl sulfide n, butane
iso-butane
propylene
propane
D6667 – 04 (2010)
TABLE 2 Typical Operating Conditions
Sample inlet system temperature 85 6 20°C
Sample injection system carrier gas 25–30 mL/min
Furnace temperature 1075 6 25°C
Furnace oxygen flow meter setting 375–450 mL/min
Inlet oxygen flow meter setting 10–30 mL/min
Inlet carrier flow meter setting 130–160 mL/min
Gas sample size 10–20 mL
LPG sample size 15 µL
TABLE 3 Typical Sulfur Calibration Ranges and Standard
Concentrations
Curve I Curve II
Sulfur mg/kg Sulfur mg/kg
Blank Blank
5.00 10.00
10.00 50.00
100.00
TABLE 4 Repeatability (r) and Reproducibility (R)
Concentration
rR
(mg/kg S)
1 0.1 0.3
5 0.6 1.6
10 1.2 3.1
25 2.9 7.8
50 5.8 15.6
100 11.5 31.3
10.3 Obtain a quantitative measurement of the injected material by filling the sample loop of the sample valve system for the
matrix being analyzed (see Table 2) (Notes 5 and 6).
NOTE 5—Injection of a constant or similar sample size for all materials analyzed in a selected operating range promotes consistent combustion
conditions and may simplify result calculations.
NOTE 6—An automatic sample transfer and injection device may be used.
10.3.1 Flush the sample loop with sufficient calibrant to assure that the material to be injected is representative.
10.3.2 For LPG samples, if bubbles are present in the viewable portion of the liquid column, flush the sample loop to introduce
a new liquid-full sample portion.
10.4 Start the analyzer and inject the calibration material according to the manufacturer’s instructions.
10.5 Calibrate the instrument using one of the following techniques.
10.5.1 Multi-point Calibration:
10.5.1.1 Whentheapparatusfeaturesaninternalself-calibrationroutine,analyzethecalibrationstandardsandblankthreetimes
using the procedures described in 10.2-10.4.
10.5.1.2 Calibrate the analyzer according to the manufacturer’s instructions to yield sulfur concentration (see Section 13). This
curve is typically linear and system performance shall be checked at least once per day, each day of use. (Note 7).
NOTE 7—Other calibration curve techniques may be used when accuracy and precision are not degraded. The frequency of calibration may be
determined by the use of quality control charts or other quality assurance/quality control techniques.
10.5.2 One-point Calibration:
10.5.2.1 Utilize a calibration standard (6.4) with a sulfur content close to that of the samples to be analyzed (625 % max.).
10.5.2.2 Follow the instrument manufacturer’s instructions to establish an instrument zero (instrument blank) by conducting an
analysis run without injection of the calibration standard.
10.5.2.3 Perform measurements of the calibration standard a minimum of three times.
10.5.2.4 Calculate a calibration factor K, in counts per nanogram of sulfur (counts/ng S) as described in 12.2.
11. Procedure
11.1 Obtain a test specimen using the procedure described in Section 8. Typically the sulfur concentration in the test specimen
is less than
...








Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.