ASTM D1142-95(2006)
(Test Method)Standard Test Method for Water Vapor Content of Gaseous Fuels by Measurement of Dew-Point Temperature
Standard Test Method for Water Vapor Content of Gaseous Fuels by Measurement of Dew-Point Temperature
SIGNIFICANCE AND USE
Generally, contracts governing the pipeline transmission of natural gas contain specifications limiting the maximum concentration of water vapor allowed. Excess water vapor can cause corrosive conditions, degrading pipelines and equipment. It can also condense and freeze or form methane hydrates causing blockages. Water–vapor content also affects the heating value of natural gas, thus influencing the quality of the gas. This test method permits the determination of water content of natural gas.
SCOPE
1.1 This test method covers the determination of the water vapor content of gaseous fuels by measurement of the dew-point temperature and the calculation therefrom of the water vapor content. Note 1Some gaseous fuels contain vapors of hydrocarbons or other components that easily condense into liquid and sometimes interfere with or mask the water dew point. When this occurs, it is sometimes very helpful to supplement the apparatus in with an optical attachment that uniformly illuminates the dew-point mirror and also magnifies the condensate on the mirror. With this attachment it is possible, in some cases, to observe separate condensation points of water vapor, hydrocarbons, and glycolamines as well as ice points. However, if the dew point of the condensable hydrocarbons is higher than the water vapor dew point, when such hydrocarbons are present in large amounts, they may flood the mirror and obscure or wash off the water dew point. Best results in distinguishing multiple component dew points are obtained when they are not too closely spaced.
Note 2
Condensation of water vapor on the dew-point mirror may appear as liquid water at temperatures as low as 0 to 10F (18 to 23C). At lower temperatures an ice point rather than a water dew point likely will be observed. The minimum dew point of any vapor that can be observed is limited by the mechanical parts of the equipment. Mirror temperatures as low as 150F (100C) have been measured, using liquid nitrogen as the coolant with a thermocouple attached to the mirror, instead of a thermometer well.
This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: D1142 − 95(Reapproved 2006)
Standard Test Method for
Water Vapor Content of Gaseous Fuels by
Measurement of Dew-Point Temperature
This standard is issued under the fixed designation D1142; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
This standard has been approved for use by agencies of the Department of Defense.
1. Scope saturated with the gas mixture. When a gas containing water
vapor is at the water dew-point temperature, it is said to be
1.1 This test method covers the determination of the water
saturated at the existing pressure.
vapor content of gaseous fuels by measurement of the dew-
point temperature and the calculation therefrom of the water 2.1.2 specific volume—of a gaseous fuel, the volume of the
gas in cubic feet per pound.
vapor content.
2.1.3 water dew-point temperature— of a gaseous fuel, the
NOTE 1—Some gaseous fuels contain vapors of hydrocarbons or other
temperature at which the gas is saturated with water vapor at
components that easily condense into liquid and sometimes interfere with
or mask the water dew point. When this occurs, it is sometimes very
the existing pressure.
helpful to supplement the apparatus in Fig. 1 with an optical attachment
that uniformly illuminates the dew–point mirror and also magnifies the
3. Significance and Use
condensate on the mirror. With this attachment it is possible, in some
cases, to observe separate condensation points of water vapor,
3.1 Generally,contractsgoverningthepipelinetransmission
hydrocarbons,andglycolaminesaswellasicepoints.However,ifthedew
of natural gas contain specifications limiting the maximum
point of the condensable hydrocarbons is higher than the water vapor dew
concentration of water vapor allowed. Excess water vapor can
point, when such hydrocarbons are present in large amounts, they may
flood the mirror and obscure or wash off the water dew point. Best results causecorrosiveconditions,degradingpipelinesandequipment.
in distinguishing multiple component dew points are obtained when they
It can also condense and freeze or form methane hydrates
are not too closely spaced.
causing blockages. Water–vapor content also affects the heat-
NOTE 2—Condensation of water vapor on the dew-point mirror may
ingvalueofnaturalgas,thusinfluencingthequalityofthegas.
appear as liquid water at temperatures as low as 0 to−10°F (−18
This test method permits the determination of water content of
to−23°C). At lower temperatures an ice point rather than a water dew
point likely will be observed. The minimum dew point of any vapor that natural gas.
can be observed is limited by the mechanical parts of the equipment.
Mirror temperatures as low as−150°F (−100°C) have been measured,
4. Apparatus
using liquid nitrogen as the coolant with a thermocouple attached to the
mirror, instead of a thermometer well.
4.1 Any properly constructed dew-point apparatus may be
1.2 This standard does not purport to address all of the used that satisfies the basic requirements that means must be
provided:
safety concerns, if any, associated with its use. It is the
responsibility of the user of this standard to establish appro- 4.1.1 To permit a controlled flow of gas to enter and leave
priate safety and health practices and determine the applica- the apparatus while the apparatus is at a temperature at least
bility of regulatory limitations prior to use. 3°F above the dew point of the gas.
4.1.2 To cool and control the cooling rate of a portion
2. Terminology
(preferably a small portion) of the apparatus, with which the
flowing gas comes in contact, to a temperature low enough to
2.1 Definitions of Terms Specific to This Standard:
cause vapor to condense from the gas.
2.1.1 saturated water vapor or equilibrium water–vapor
4.1.3 To observe the deposition of dew on the cold portion
content—the water vapor concentration in a gas mixture that is
of the apparatus.
in equilibrium with a liquid phase of pure water that is
4.1.4 To measure the temperature of the cold portion on the
apparatus on which the dew is deposited, and
4.1.5 To measure the pressure of the gas within the appara-
ThistestmethodisunderthejurisdictionofASTMCommitteeD03onGaseous
Fuels and is the direct responsibility of Subcommittee D03.05 on Determination of
tus or the deviation from the known existing barometric
Special Constituents of Gaseous Fuels.
pressure.
Current edition approved June 1, 2006. Published June 2006. Originally
4.1.6 The apparatus should be constructed so that the “cold
approved in 1950. Last previous edition approved in 2000 as D1142–95(2000).
DOI: 10.1520/D1142-95R06. spot,” that is, the cold portion of the apparatus on which dew
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
D1142 − 95 (2006)
FIG. 1 Bureau of Mines Dew-Point Apparatus
is deposited, is protected from all gases other than the gas either end. The lower header is connected with the upper
under test. The apparatus may or may not be designed for use
header by numerous small holes drilled in the copper body
under pressure. through which the vaporized refrigerant passes. The chiller is
attached to the cooling rod, F, by means of a taper joint. The
4.2 The Bureau of Mines type of dew-point apparatus
temperature of the target mirror, C, is indicated by a calibrated
shown in Fig. 1 fulfills the requirements specified in 4.1.
mercury-in-glass thermometer, K, whose bulb fits snugly into
Within the range of conditions in Section 1, this apparatus is
the thermometer well. Observation of the dew deposit is made
satisfactory for determining the dew point of gaseous fuels.
through the pressure-resisting transparent window, E.
Briefly, this apparatus consists of a metal chamber into and out
4.2.1 Note that only the central portion of the stainless steel
ofwhichthetestgasispermittedtoflowthroughcontrolvalves
A and D. Gas entering the apparatus through valve A is target mirror, C, is thermally bonded to the fitting, I, through
deflectedbynozzleBtowardsthecoldportionoftheapparatus, which C is cooled. Since stainless steel is a relatively poor
C. The gas flows across the face of C and out of the apparatus thermal conductor, the central portion of the mirror is thus
through valve D. Part C is a highly polished stainless steel maintained at a slightly lower temperature than the outer
“target mirror,” cooled by means of a copper cooling rod, F. portion, with the result that the dew first appears on the central
The mirror, C, is silver-soldered to a nib on the copper portionofthemirroranditsdetectionisaidedmateriallybythe
thermometer well fitting, I, which is soft-soldered to the
contrast afforded.The arrangement for measuring the tempera-
cooling rod, F. The thermometer well is integral with the ture of the target mirror, C, also should be noted. The
fitting, I. Cooling of rod F is accomplished by vaporizing a
temperature is read with a thermometer or RTD, K, inserted in
refrigerant such as liquid butane, propane, carbon dioxide, or the cooling rod, F, so that the bulb of the temperature
some other liquefied gas in the chiller, G. The refrigerant is
measuring device is entirely within the thermometer well in
throttled into the chiller through valve H and passes out at J.
fitting, I. The stud to which the stainless steel mirror is
The chiller body is made of copper and has brass headers on
silver-soldered is a part of the base of the thermometer well,
and as there is no metallic contact between the thermometer
well and the cooling tube, other than through its base, the
Deaton, W. M., and Frost, E. M., Jr., “Bureau of Mines Apparatus for
thermometer or RTD indicates the temperature of the mirror
Determining the Dew Point of Gases Under Pressure,” Bureau of Mines Report of
Investigation 3399, May 1938. rather than some compromise temperature influenced by the
D1142 − 95 (2006)
temperature gradient along the cooling tube as would be the 0.2°F (0.1°C) allow equilibrium conditions to be approached
case if this type of construction were not used. The RTD will closely and favor an accurate determination. When dew has
include suitable electronics and display. been deposited, allow the target mirror to warm up at a rate
4.2.2 Tests with the Bureau of Mines type of dew-point comparable to the recommended rate of cooling. The normal
apparatus are reported to permit a determination with a warming rate usually will be faster than desired. To reduce the
precision (reproducibility) of 60.2°F (60.1°C) and with an rate, “crack” valve H momentarily at intervals to supply
accuracy of 60.2°F (60.1°C) when the dew-point tempera- coolingtothecoolingtube, F.Repeatthecoolingandwarming
tures range from room temperature to a temperature of 32°F cycles several times. The arithmetic average of the tempera-
(0°C). It is estimated that water dew points may be determined tures at which dew is observed to appear and disappear is
with an accuracy of 60.5°F (0.3°C) when they are below 32°F considered to be the observed dew point.
(0°C) and not lower than 0°F (−17.8°C), provided ice crystals
NOTE 3—If the water–vapor content is to be calculated as described in
do not form during the determination.
6.2,thegasspecimenshouldbethrottledattheinletvalve, A,toapressure
within the apparatus approximately equal to atmospheric pressure. The
5. Procedure
outlet valve may be left wide open or restricted, as desired. The pressure
existing within the apparatus must, however, be known to the required
5.1 General Considerations—Take the specimen so as to be
accuracy.
representative of the gas at the source. Do not take at a point
where isolation would permit condensate to collect or would
6. Calculation
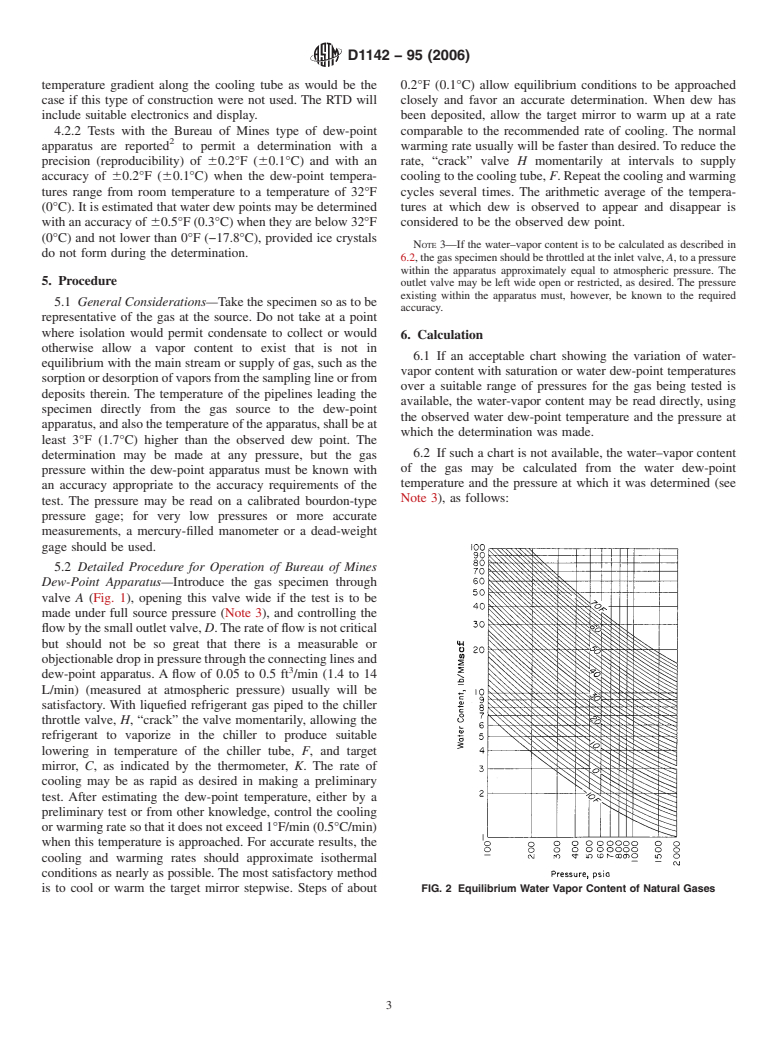
otherwise allow a vapor content to exist that is not in
6.1 If an acceptable chart showing the variation of water-
equilibrium with the main stream or supply of gas, such as the
vapor content with saturation or water dew-point temperatures
sorptionordesorptionofvaporsfromthesamplinglineorfrom
over a suitable range of pressures for the gas being tested is
deposits therein. The temperature of the pipelines leading the
available, the water-vapor content may be read directly, using
specimen directly from the gas source to the dew-point
the observed water dew-point temperature and the pressure at
apparatus,andalsothetemperatureoftheapparatus,shallbeat
which the determination was made.
least 3°F (1.7°C) higher than the observed dew point. The
6.2 If such a chart is not available, the water–vapor content
determination may be made at any pressure, but the gas
of the gas may be calculated from the water dew-point
pressure within the dew-point apparatus must be known with
temperature and the pressure at which it was determined (see
an accuracy appropriate to the accuracy requirements of the
Note 3), as follows:
test. The pressure may be read on a calibrated bourdon-type
pressure gage; for very low pressures or more accurate
measurements, a mercury-filled manometer or a dead-weight
gage should be used.
5.2 Detailed Procedure for Operation of Bureau of Mines
Dew-Point Apparatus—Introduce the gas specimen through
valve A (Fig. 1), opening this valve wide if the test is to be
made under full source pressure (Note 3), and controlling the
flowbythesmalloutletvalve, D.Therateofflowisnotcritical
but should not be so great that there is a measurable or
objectionabledropinpressurethroughtheconnectinglinesand
dew-point apparatus. A flow of 0.05 to 0.5 ft /min (1.4 to 14
L/min) (measured at atmospheric pressure) usually will be
satisfactory. With liquefied refrigerant gas piped to the chiller
throttle valve, H, “crack” the valve momentarily, allowing the
refrigerant to vaporize in the chiller to produce suitable
lowering in temperature of the chiller tube, F, and target
mirror, C, as indicated by the thermometer, K. The rate of
cooling may be as rapid as desired in making a preliminary
test. After estimating the dew-point temperature, either by a
preliminary test or from other knowledge, control the cooling
orwarmingratesothatitdoesnotexceed1°F/min(0.5°C/min)
when this temperature is approached. For accurate results, the
cooling and warming rates should approximate isothermal
conditions as nearly as possible. The most satisfactory method
is to cool or warm the target mirror stepwise. Steps of about FIG. 2 Equilibrium Water Vapor Content of Natural Gases
D1142 − 95 (2006)
W 5 w 310 3 P /P 3 T/T (1) 6.3 A correlation of the available data on the equilibrium
~ ~ !!
b b
water content of natural gases has been reported by Bukacek.
where:
This correlation is believed to be accurate enough for the
W = lb of water/million ft of gaseous mixture at pressure
requirements of the gaseous fuels industry, except for unusual
P and temperature T ;
b b situations where the dew point is measured at conditions close
w = weight of saturated water vapor, lb/ft , at the water
to the critical temperature of the gas. The correlation is a
dew-point temperature, that is, the reciprocal of the
modified form of Raoult’s law having the following form:
specific volume of saturated vapor (see Table 1);
W 5 A/P 1B (2)
~ !
P = pressure-base of gas measurement, psia;
b
P = pressure at which the water dew point of gas was
where:
determined, psia;
W = water–vapor content, lb/million ft ;
t = observed water dew-point temperature, °F;
P = total pressure, psia;
T = Rankine (absolute Fahrenheit scale) water dew point, t
A = a constant proportional to the vapor pressure of water;
+460, at pressure P; and
and
T = base temperature of gas measurement, t +460.
b b
B = a constant depending on temperature and gas
NOTE 4—Example 1:
composition.
Given: Water dew point=37°F at 15.0-psia pressure.
NOTE 5—Values of B were computed from available data on methane,
What is the water–vapor content million ft of gas (gas measurement
methane-ethane mixtures, and natural gases.
base of 60°F and 14.7-psia pressure)?
6.3.1 Table2listsvaluesoftheconstantsAandBfornatural
From Table 1 the specific volume of saturated water at 37°F is 2731.9
gases in the temperature range from−40 to 460°F (−40 to
ft /lb, from which:
w =(1/2731.9)=0.0003660 lb/ft
238°C).
and
6.3.2 Tables 3-5 list values of water–vapor content
W =0.0003660×10 ×(14.7/15.0)×[(460+37)/(460+60)]
from−40to250°F(−40°to121°C)andfrom14.7to5000psia
=342.8 lb/million ft
(101 to 34475 kPa), covering the range of most natural gas
Example 2:
processing applications.
Given: Wat
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.