ASTM D3712-05(2017)
(Test Method)Standard Test Method of Analysis of Oil-Soluble Petroleum Sulfonates by Liquid Chromatography
Standard Test Method of Analysis of Oil-Soluble Petroleum Sulfonates by Liquid Chromatography
SIGNIFICANCE AND USE
5.1 This test method provides a means of determining sulfonate content and of classifying and characterizing natural and synthetic petroleum sulfonate products by sulfonate content and average molecular weight. Purity of sodium sulfonate products is measured by basicity and inorganic salt contents and the reserve alkalinity of alkaline earth sulfonates by the total base number.
SCOPE
1.1 This test method covers the analysis of refined and crude natural and synthetic oil-soluble sulfonate products. Resins, if present, are recovered with the oil phase and carboxylates are recovered as sulfonates.
1.2 This test method covers the determination of mineral oil, sodium sulfonate, inorganic salts, water, basicity or acidity, average molecular weight, and relative density of sodium sulfonate products.
1.3 This test method covers the determination of mineral oil, sulfonate, water, base number, average molecular weight, and relative density of calcium, barium, magnesium, and ammonium sulfonate products.
1.4 The values stated in SI units are to be regarded as the standard.
1.4.1 Exception—The values given in parentheses are for information only.
1.5 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
1.6 This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
General Information
Relations
Buy Standard
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: D3712 − 05 (Reapproved 2017)
Designation: 369/(95)
Standard Test Method of
Analysis of Oil-Soluble Petroleum Sulfonates by Liquid
Chromatography
This standard is issued under the fixed designation D3712; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 2. Referenced Documents
1.1 Thistestmethodcoverstheanalysisofrefinedandcrude 2.1 ASTM Standards:
natural and synthetic oil-soluble sulfonate products. Resins, if D95Test Method for Water in Petroleum Products and
present, are recovered with the oil phase and carboxylates are Bituminous Materials by Distillation
recovered as sulfonates. D2896TestMethodforBaseNumberofPetroleumProducts
by Potentiometric Perchloric Acid Titration
1.2 This test method covers the determination of mineral
oil,sodiumsulfonate,inorganicsalts,water,basicityoracidity,
3. Terminology
average molecular weight, and relative density of sodium
3.1 Symbols:
sulfonate products.
3.1.1 Following are definitions of the symbols used in
1.3 This test method covers the determination of mineral
Section 17, and as noted in the sections in parentheses.
oil, sulfonate, water, base number, average molecular weight,
A = grams of sample of calcium, barium, magnesium, or ammonium
and relative density of calcium, barium, magnesium, and
sulfonate (8.1.1).
B = volume of chloroform solution, mL (10.1).
ammonium sulfonate products.
C = grams of sample of sodium sulfonate (10.1.1).
1.4 The values stated in SI units are to be regarded as the
D = grams of oil recovered (10.4).
E = grams of sodium sulfonate recovered (10.5).
standard.
F = grams of residue from chloroform blank (10.6).
1.4.1 Exception—The values given in parentheses are for
G = grams of residue from alcohol blank (10.6).
information only.
H = grams of sodium sulfonate (11.1).
I = grams of sodium sulfate ash from sodium sulfonate (11.2).
1.5 This standard does not purport to address all of the
J = T/KS.
safety concerns, if any, associated with its use. It is the
K = valence of cation.
S = average equivalent weight of sodium sulfonate (17.1.4).
responsibility of the user of this standard to establish appro-
T = average molecular weight of calcium, barium, magnesium, or
priate safety and health practices and determine the applica-
ammonium sulfonate (17.1.5).
bility of regulatory limitations prior to use.
U = percentage of sodium sulfonate (17.1.2).
V = percentage of calcium, barium, magnesium, or ammonium
1.6 This international standard was developed in accor-
sulfonate (17.1.3).
dance with internationally recognized principles on standard-
W = grams of water contained in pycnometer at 25 °C (6.9).
c
ization established in the Decision on Principles for the
W = grams of sample contained in pycnometer at 25 °C (15.1).
s
X = grams of sodium sulfonate sample for basicity (12.1).
Development of International Standards, Guides and Recom-
Y = volume of standard H SO or NaOH solution used to determine
2 4
mendations issued by the World Trade Organization Technical
basicity or acidity (12.1).
Barriers to Trade (TBT) Committee. Z = normality of standard H SO or NaOH solution to determine free
2 4
basicity or acidity (12.1).
AA = grams of sodium sulfonate product ashed (13.1).
BB = grams of sodium sulfate from inorganic salt determination (13.1).
This test method is under the jurisdiction of ASTM Committee D02 on
Petroleum Products, Liquid Fuels, and Lubricantsand is the direct responsibility of
Subcommittee D02.04.0C on Liquid Chromatography.
Current edition approved July 15, 2017. Published July 2017. Originally For referenced ASTM standards, visit the ASTM website, www.astm.org, or
approved in 1978. Last previous edition approved in 2011 as D3712–05 (2011). contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
DOI: 10.1520/D3712-05R17. Standards volume information, refer to the standard’s Document Summary page on
This test method was adopted as a joint ASTM-IP standard. the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
D3712 − 05 (2017)
CC = percentage of free acidity of sodium sulfonate product as H SO
A 2 4
(17.1.6).
CC = percentage of free basicity of sodium sulfonate product as NaOH
B
(17.1.6).
DD = percentage of inorganic salts as sodium sulfate (17.1.7).
4. Summary of Test Method
4.1 The sample, except a sodium sulfonate product, is
dissolved in ethyl ether and converted to sulfonic acid, using
dilute hydrochloric acid. The sulfonic acid after extraction is
converted to sodium sulfonate and the isolated sodium sul-
fonate and mineral oil are dissolved in chloroform.An aliquot
of the chloroform solution, or a sample of a sodium sulfonate
product, dissolved in chloroform, is placed on a silica gel
column. The oil is eluted with chloroform, the sulfonate with
ethyl alcohol, and both are determined gravimetrically. Aver-
agemolecularweightiscalculatedfromtheaverageequivalent
weightofthesodiumsulfonate,whichisdeterminedbyashing
a portion of the isolated sodium sulfonate.
4.2 Water is determined by Test Method D95. Base number
is determined by Test Method D2896. Relative density is
determined by pycnometer.
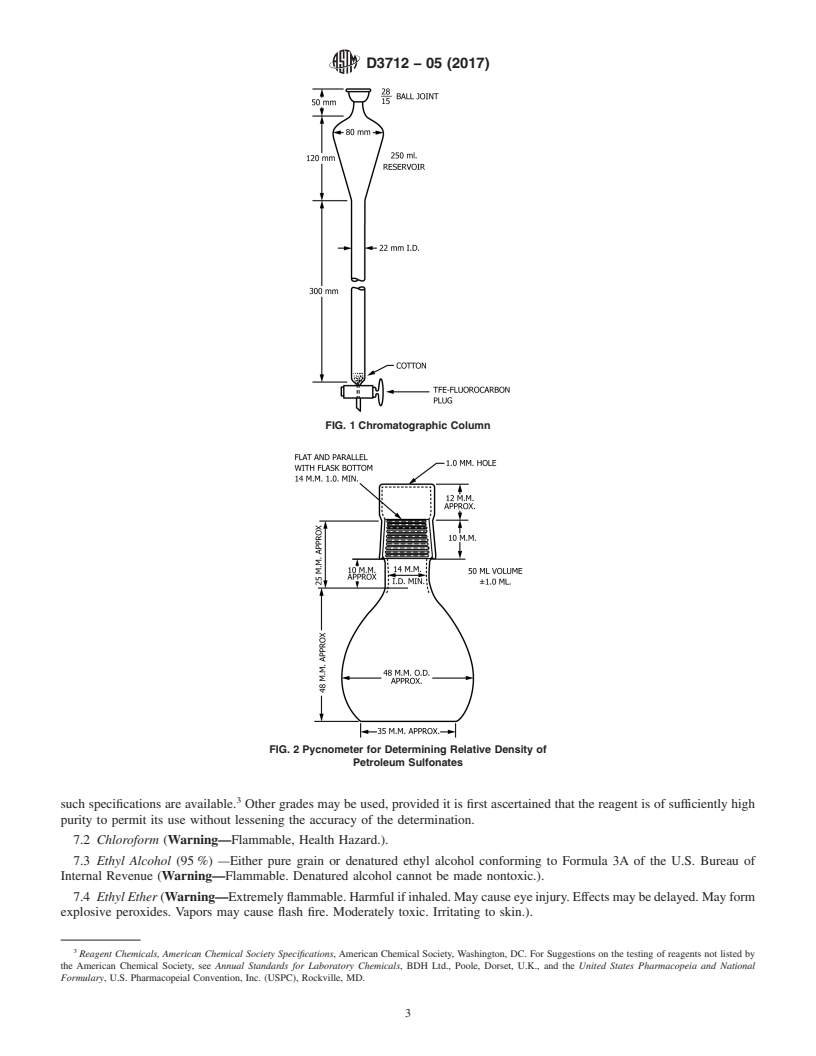
FIG. 1 Chromatographic Column
5. Significance and Use
5.1 This test method provides a means of determining
sulfonate content and of classifying and characterizing natural
and synthetic petroleum sulfonate products by sulfonate con-
tent and average molecular weight. Purity of sodium sulfonate
products is measured by basicity and inorganic salt contents
and the reserve alkalinity of alkaline earth sulfonates by the
total base number.
6. Apparatus
6.1 Chromatographic column, made of glass and consisting
of a reservoir and separator section, and fitted with a TFE-
fluorocarbonstopcockwitha2mmbore,asshowninFig.1.A
column with a detachable reservoir connected by a standard-
taper joint can be used.
6.2 Steam Bath.
6.3 Vacuum Desiccator, shielded.
6.4 Vacuum Oven, capable of being maintained at 100°C
(212°F)andconnectedto559mmto635mm(22in.to29in.)
Hg vacuum.
FIG. 2 Pycnometer for Determining Relative Density of
6.5 Muffle Furnace, capable of operating at 800°C to Petroleum Sulfonates
1000°C (1500°F to 1800°F).
6.6 Dish, platinum, 100mL capacity.
obtainaflatmeniscus,addaminuteamountofwettingagentto
6.7 Distillation Apparatus, as described in Test Method
the water surface. Remove the pycnometer from the bath, and
D95.
drytheoutside.Replacethecapandweightothenearest1mg.
6.8 Water Bath, capable of being maintained at 25°C 6
Record the mass of water contained as W .
c
0.2°C (77°F 6 0.3°F).
7. Reagents and Materials
6.9 Pycnometer, as shown in Fig. 2. To calibrate, weigh to
thenearest1mgwithcapinplace;thenfillwithdistilledwater 7.1 Purity of Reagents—Reagent grade chemicals shall be
at 15°C to 20°C (60°F to 68°F) and place in water bath at used in all tests. Unless otherwise indicated, it is intended that
25°C 6 0.2°C (77°F 6 0.3°F). After 30min, adjust the all reagents shall conform to the specifications of the Commit-
water meniscus at the top of the neck so it is exactly level. To tee onAnalytical Reagents of theAmerican Chemical Society,
D3712 − 05 (2017)
where such specifications are available. Other grades may be fire. May be fatal if swallowed. Liquid and vapor cause severe
used, provided it is first ascertained that the reagent is of burns. Harmful if inhaled. Contact with water liberates large
sufficiently high purity to permit its use without lessening the
amounts of heat. Spillage may cause fire.
accuracy of the determination.
7.14.1 Sulfuric Acid Solution, Standard (0.1 mol⁄L)—
Prepare and standardize a 0.1mol⁄L aqueous sulfuric acid
7.2 Chloroform (Warning—Flammable, Health Hazard.).
(H SO ).
2 4
7.3 Ethyl Alcohol (95%) —Either pure grain or denatured
ethyl alcohol conforming to Formula 3Aof the U.S. Bureau of
8. Conversion of Calcium, Barium, Magnesium, or
Internal Revenue (Warning—Flammable. Denatured alcohol
Ammonium Sulfonate to Sodium Sulfonate
cannot be made nontoxic.).
8.1 Conversion of Calcium, Barium, Magnesium or Ammo-
7.4 Ethyl Ether(Warning—Extremelyflammable.Harmful
nium Sulfonate to Sulfonic Acid:
if inhaled. May cause eye injury. Effects may be delayed. May
form explosive peroxides. Vapors may cause flash fire. Mod- 8.1.1 Transferapproximately10gofsample,weighedtothe
erately toxic. Irritating to skin.).
nearest 0.001g into a 250mL Erlenmeyer flask, designating
this weight as A.Add 50mLof ethyl ether and stir to dissolve
7.5 Filter Paper, slow-filtering, ashless, gravimetric.
thesample.Add100mLofdiluteHCl(1+1)andswirltomix
7.6 Hydrochloric Acid (Concentrated)—(Warning—
thoroughly until reaction is complete. In analyzing barium
Poison. Corrosive. May be fatal if swallowed. Liquid and
sulfonate products if barium chloride crystallizes out, add
vapor cause severe burns. Harmful if inhaled.).
sufficient water to redissolve.
7.6.1 Hydrochloric Acid, Dilute (1 + 1) —(See Warning in
8.1.2 Quantitatively transfer the mixture to a 500mL sepa-
7.6.) One volume of concentrated hydrochloric acid (HCl) is
ratory funnel. Shake well, let settle, and draw the aqueous acid
added to 1 volume of water.
layerintoa250mLseparatoryfunnel.Extracttheaqueousacid
7.6.2 Hydrochloric Acid, Dilute (1 + 3) —(See Warning in
layer in the 250mL separatory funnel with three 50mL
7.6.) One volume of concentrated hydrochloric acid (HCl) is
portionsofethylether,usingtheethyletherwashestorinsethe
added to 3 volumes of water.
Erlenmeyer flask first. Combine all the ethyl ether extracts in
7.7 Isopropyl Alcohol (99% by Mass)—Water content shall
the 500mL separatory funnel and wash with 50mL of dilute
be 0.9% by mass maximum. (Warning—Flammable.)
HCl (1+3). Combine all the aqueous acid layers and reextract
7.7.1 Isopropyl Alcohol, Dilute (1+1) —One volume of
them with 50mL of ethyl ether.
99% by mass isopropyl alcohol is diluted with 1 volume of
water. 8.2 Conversion of Sulfonic Acid to Sodium Sulfonate:
8.2.1 Collect all of the ether washes in the 500mL separa-
7.8 Methyl Orange Indicator Solution—Dissolve 1.0g of
tory funnel and shake with successive 50mL portions of
methyl orange in water and dilute to 1L.
Na SO solution containing 2 to 3 drops of methyl orange
2 4
7.9 Phenolphthalein Indicator Solution—Dissolve 1g of
indicatoruntilawashingdoesnotappearpink.Discardthesalt
phenolphthalein in 100mL of 50% by mass ethyl alcohol.
washes.
7.10 Silica Gel, 250µm to 74µm (60mesh to 200mesh).
8.2.2 Drain off as much of the aqueous layer as possible
7.11 Sodium Hydroxide Solution, Standard (0.1mol⁄L) from the washed ether solution. Lay the separatory funnel on
(Warning—Corrosive. Can cause severe burns or blindness.
its side and introduce about 10g of anhydrous Na SO .
2 4
Evolution of heat produces a violent reaction or eruption upon
Stopperthefunnel,makingsurethatthefunnelmouthisfreeof
too rapid mixture with water.)—Prepare and standardize a
Na SO crystals and shake the mixture vigorously for 3min to
2 4
0.1mol⁄L aqueous, carbonate-free, NaOH solution.
4min, to remove the last traces of water, venting the funnel
frequently. Place a 250mL Erlenmeyer flask on a steam bath
7.12 Sodium Sulfate, Anhydrous, Crystalline.
and filter the ether solution through a small plug of cotton,
7.13 Sodium Sulfate Solution—Dissolve 240g of sodium
placedinthevortexofafilterfunnel,intotheErlenmeyerflask,
sulfate (Na SO ) in water and dilute to 1L.
2 4
keeping approximately 50mL of solution in the Erlenmeyer
7.14 Sulfuric Acid (relative density 1.84)—Concentrated
flaskwhileevaporating.Rinsethefunnelandfilterwith50mL
sulfuric acid (H SO ), 36mol⁄L. (Warning—Poison. Corro-
2 4 of ethyl ether, adding the rinsing to the main ether solution.
sive. Strong oxidizer. Contact with organic material may cause
Evaporate the ethyl ether until approximately 10mL of solu-
tion remains. Add 50mL of 99% by mass isopropyl alcohol,
several drops of phenolphthalein indicator solution, and titrate
Reagent Chemicals, American Chemical Society Specifications, American
Chemical Society, Washington, DC. For Suggestions on the testing of reagents not with 0.1mol⁄L standard NaOH solution to the red color
listed by the American Chemical Society, see Annual Standards for Laboratory
change. Place the flask on a steam bath and evaporate to
Chemicals, BDH Ltd., Poole, Dorset, U.K., and the United States Pharmacopeia
dryness. Dissolve the sodium sulfonate and oil residue in
and National Formulary, U.S. Pharmacopeial Convention, Inc. (USPC), Rockville,
chloroform; transfer quantitatively into a 100mL volumetric
MD.
The sole source of supply of silica gel, Grade 62, known to the committee at
flask, adjust to volume, and proceed directly with Section 10.
this time is W.R. Grace and Co., Davison Chemical Corp., Baltimore, MD 21203.
The solution may turn acidic on standing in the laboratory.
If you are aware of alternative suppliers, please provide this information toASTM
Should this occur, add sufficient 0.1 N NaOH solution to the
International Headquarters. Your comments will receive careful consideration at a
meeting of the responsible technical committee, which you may attend. aliquot taken until the solution is pink.
D3712 − 05 (2017)
9. Preparation of the Column 10.3 Elution of Sulfonate—Tare a second 250mLbeaker to
the nearest 0.0001g and place it under the column. Add
9.1 With the stopcock closed, pour 80mL to 100mL of
230mLofethylalcoholtothereservoir,openthestopcockand
chloroform into the column, and push a wad of cotton to the
adjust the flow rate as described in 10.2.1 (Note 2). When the
bottom with a rod (Note 1). Compress the cotton enough to
receivingbeakerisabouthalffull,removeitandplaceaclean,
hold back the silica gel but not enough to impede the flow of
untared 250mL beaker under the column. Place the tared
solvent.
beakeronthesteambathandevaporategently,blowingfiltered
NOTE1—Acoarse-fritteddiskmadeofborosilicateglasscanbeusedin
airovertheliquidsurface.Whentheliquidlevelinthecolumn
place of the cotton wad.
is within 13mm ( ⁄2in.) of the surface of the gel, close the
9.2 Pour15g 61gofsilicagelintothecolumncontaining
stopcockandquantitativelytransferthecontentsoftheuntared
the chloroform. The column must be free of air bubbles to
beakertothesecondtaredbeakeronthesteambath,bytheuse
avoid channeling. Start the flow of chloroform by opening the
of ethyl alcohol.
stopcock.When the liquid level is within 13mm ( ⁄2in.) of the
NOTE 2—The flow rat
...
This document is not an ASTM standard and is intended only to provide the user of an ASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
Designation: D3712 − 05 (Reapproved 2011) D3712 − 05 (Reapproved 2017)
Designation: 369/(95)
Standard Test Method of
Analysis of Oil-Soluble Petroleum Sulfonates by Liquid
Chromatography
This standard is issued under the fixed designation D3712; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope
1.1 This test method covers the analysis of refined and crude natural and synthetic oil-soluble sulfonate products. Resins, if
present, are recovered with the oil phase and carboxylates are recovered as sulfonates.
1.2 This test method covers the determination of mineral oil, sodium sulfonate, inorganic salts, water, basicity or acidity,
average molecular weight, and relative density of sodium sulfonate products.
1.3 This test method covers the determination of mineral oil, sulfonate, water, base number, average molecular weight, and
relative density of calcium, barium, magnesium, and ammonium sulfonate products.
1.4 The values stated in SI units are to be regarded as the standard. The values given in parentheses are for information only.
1.4.1 Exception—The values given in parentheses are for information only.
1.5 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility
of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory
limitations prior to use.
1.6 This international standard was developed in accordance with internationally recognized principles on standardization
established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued
by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
2. Referenced Documents
2.1 ASTM Standards:
D95 Test Method for Water in Petroleum Products and Bituminous Materials by Distillation
D2896 Test Method for Base Number of Petroleum Products by Potentiometric Perchloric Acid Titration
3. Terminology
3.1 Symbols:
3.1.1 Following are definitions of the symbols used in Section 17, and as noted in the sections in parentheses.
A = grams of sample of calcium, barium, magnesium, or ammonium
sulfonate (8.1.1).
B = volume of chloroform solution, mL (10.1).
C = grams of sample of sodium sulfonate (10.1.1).
D = grams of oil recovered (10.4).
E = grams of sodium sulfonate recovered (10.5).
F = grams of residue from chloroform blank (10.6).
G = grams of residue from alcohol blank (10.6).
H = grams of sodium sulfonate (11.1).
I = grams of sodium sulfate ash from sodium sulfonate (11.2).
J = T/KS.
This test method is under the jurisdiction of ASTM Committee D02 on Petroleum Products, Liquid Fuels, and Lubricantsand is the direct responsibility of Subcommittee
D02.04.0C on Liquid Chromatography.
Current edition approved Jan. 1, 2011July 15, 2017. Published February 2011July 2017. Originally approved in 1978. Last previous edition approved in 20052011 as
D3712 – 05.D3712 – 05 (2011). DOI: 10.1520/D3712-05R11.10.1520/D3712-05R17.
This test method was adopted as a joint ASTM-IP standard.
For referenced ASTM standards, visit the ASTM website, www.astm.org, or contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM Standards
volume information, refer to the standard’s Document Summary page on the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
D3712 − 05 (2017)
K = valence of cation.
S = average equivalent weight of sodium sulfonate (17.1.4).
T = average molecular weight of calcium, barium, magnesium, or
ammonium sulfonate (17.1.5).
U = percentage of sodium sulfonate (17.1.2).
V = percentage of calcium, barium, magnesium, or ammonium
sulfonate (17.1.3).
W = grams of water contained in pycnometer at 25°C (6.9).
c
W = grams of water contained in pycnometer at 25 °C (6.9).
c
W = grams of sample contained in pycnometer at 25°C (15.1).
s
W = grams of sample contained in pycnometer at 25 °C (15.1).
s
X = grams of sodium sulfonate sample for basicity (12.1).
Y = volume of standard H SO or NaOH solution used to determine
2 4
basicity or acidity (12.1).
Z = normality of standard H SO or NaOH solution to determine free
2 4
basicity or acidity (12.1).
AA = grams of sodium sulfonate product ashed (13.1).
BB = grams of sodium sulfate from inorganic salt determination (13.1).
CC = percentage of free acidity of sodium sulfonate product as H SO
A 2 4
(17.1.6).
CC = percentage of free basicity of sodium sulfonate product as NaOH
B
(17.1.6).
DD = percentage of inorganic salts as sodium sulfate (17.1.7).
4. Summary of Test Method
4.1 The sample, except a sodium sulfonate product, is dissolved in ethyl ether and converted to sulfonic acid, using dilute
hydrochloric acid. The sulfonic acid after extraction is converted to sodium sulfonate and the isolated sodium sulfonate and mineral
oil are dissolved in chloroform. An aliquot of the chloroform solution, or a sample of a sodium sulfonate product, dissolved in
chloroform, is placed on a silica gel column. The oil is eluted with chloroform, the sulfonate with ethyl alcohol, and both are
determined gravimetrically. Average molecular weight is calculated from the average equivalent weight of the sodium sulfonate,
which is determined by ashing a portion of the isolated sodium sulfonate.
4.2 Water is determined by Test Method D95. Base number is determined by Test Method D2896. Relative density is determined
by pycnometer.
5. Significance and Use
5.1 This test method provides a means of determining sulfonate content and of classifying and characterizing natural and
synthetic petroleum sulfonate products by sulfonate content and average molecular weight. Purity of sodium sulfonate products
is measured by basicity and inorganic salt contents and the reserve alkalinity of alkaline earth sulfonates by the total base number.
6. Apparatus
6.1 Chromatographic column, made of glass and consisting of a reservoir and separator section, and fitted with a
TFE-fluorocarbon stopcock with a 2-mm2 mm bore, as shown in Fig. 1. A column with a detachable reservoir connected by a
standard-taper joint can be used.
6.2 Steam Bath.
6.3 Vacuum Desiccator, shielded.
6.4 Vacuum Oven, capable of being maintained at 100°C (212°F)100 °C (212 °F) and connected to 559 to 635 mm (22 to 29
in.) 559 mm to 635 mm (22 in. to 29 in.) Hg vacuum.
6.5 Muffle Furnace, capable of operating at 800800 °C to 1000°C (15001000 °C (1500 °F to 1800°F).1800 °F).
6.6 Dish, platinum, 100-mL100 mL capacity.
6.7 Distillation Apparatus, as described in Test Method D95.
6.8 Water Bath, capable of being maintained at 2525 °C 6 0.2°C (770.2 °C (77 °F 6 0.3°F).0.3 °F).
6.9 Pycnometer, as shown in Fig. 2. To calibrate, weigh to the nearest 1 mg 1 mg with cap in place; then fill with distilled water
at 1515 °C to 20°C (6020 °C (60 °F to 68°F)68 °F) and place in water bath at 2525 °C 6 0.2°C (770.2 °C (77 °F 6 0.3°F).0.3 °F).
After 30 min, 30 min, adjust the water meniscus at the top of the neck so it is exactly level. To obtain a flat meniscus, add a minute
amount of wetting agent to the water surface. Remove the pycnometer from the bath, and dry the outside. Replace the cap and
weigh to the nearest 1 mg. Record the mass of water contained as W .
c
7. Reagents and Materials
7.1 Purity of Reagents—Reagent grade chemicals shall be used in all tests. Unless otherwise indicated, it is intended that all
reagents shall conform to the specifications of the Committee on Analytical Reagents of the American Chemical Society, where
D3712 − 05 (2017)
FIG. 1 Chromatographic Column
FIG. 2 Pycnometer for Determining Relative Density of
Petroleum Sulfonates
such specifications are available. Other grades may be used, provided it is first ascertained that the reagent is of sufficiently high
purity to permit its use without lessening the accuracy of the determination.
7.2 Chloroform (Warning—Flammable, Health Hazard.).
7.3 Ethyl Alcohol (95 %) —Either pure grain or denatured ethyl alcohol conforming to Formula 3A of the U.S. Bureau of
Internal Revenue (Warning—Flammable. Denatured alcohol cannot be made nontoxic.).
7.4 Ethyl Ether (Warning—Extremely flammable. Harmful if inhaled. May cause eye injury. Effects may be delayed. May form
explosive peroxides. Vapors may cause flash fire. Moderately toxic. Irritating to skin.).
Reagent Chemicals, American Chemical Society Specifications, American Chemical Society, Washington, DC. For Suggestions on the testing of reagents not listed by
the American Chemical Society, see Annual Standards for Laboratory Chemicals, BDH Ltd., Poole, Dorset, U.K., and the United States Pharmacopeia and National
Formulary, U.S. Pharmacopeial Convention, Inc. (USPC), Rockville, MD.
D3712 − 05 (2017)
7.5 Filter Paper, slow-filtering, ashless, gravimetric.
7.6 Hydrochloric Acid (Concentrated)—(Warning—Poison. Corrosive. May be fatal if swallowed. Liquid and vapor cause
severe burns. Harmful if inhaled.).
7.6.1 Hydrochloric Acid, Dilute (1 + 1) —(See Warning in 7.6.) One volume of concentrated hydrochloric acid (HCl) is added
to 1 volume of water.
7.6.2 Hydrochloric Acid, Dilute (1 + 3) —(See Warning in 7.6.) One volume of concentrated hydrochloric acid (HCl) is added
to 3 volumes of water.
7.7 Isopropyl Alcohol (99 Mass %)(99 % by Mass)—Water content shall be 0.9 0.9 % by mass % maximum. (Warning—
Flammable.)
7.7.1 Isopropyl Alcohol, Dilute (1 + 1) —One volume of 99 99 % by mass % isopropyl alcohol is diluted with 1 volume of
water.
7.8 Methyl Orange Indicator Solution—Dissolve 1.0 g 1.0 g of methyl orange in water and dilute to 1 L.1 L.
7.9 Phenolphthalein Indicator Solution—Dissolve 1 g 1 g of phenolphthalein in 100 mL of 50 mass % 100 mL of 50% by mass
ethyl alcohol.
7.10 Silica Gel, 250 to 74 μm (60 to 200-mesh).250 μm to 74 μm (60 mesh to 200 mesh).
7.11 Sodium Hydroxide Solution, Standard (0.1(0.1 mol mol/L) ⁄L) (Warning—Corrosive. Can cause severe burns or
blindness. Evolution of heat produces a violent reaction or eruption upon too rapid mixture with water.)—Prepare and standardize
a 0.10.1 mol mol/L ⁄L aqueous, carbonate-free, NaOH solution.
7.12 Sodium Sulfate, Anhydrous, Crystalline.
7.13 Sodium Sulfate Solution—Dissolve 240 g 240 g of sodium sulfate (Na SO ) in water and dilute to 1 L.1 L.
2 4
7.14 Sulfuric Acid (relative density 1.84)—Concentrated sulfuric acid (H SO ), 3636 mol mol/L. ⁄L. (Warning—Poison.
2 4
Corrosive. Strong oxidizer. Contact with organic material may cause fire. May be fatal if swallowed. Liquid and vapor cause severe
burns. Harmful if inhaled. Contact with water liberates large amounts of heat. Spillage may cause fire.
7.14.1 Sulfuric Acid Solution, Standard (0.1 (0.1 mol mol/L)⁄L)—Prepare and standardize a 0.10.1 mol mol/L ⁄L aqueous
sulfuric acid (H SO ).
2 4
8. Conversion of Calcium, Barium, Magnesium, or Ammonium Sulfonate to Sodium Sulfonate
8.1 Conversion of Calcium, Barium, Magnesium or Ammonium Sulfonate to Sulfonic Acid:
8.1.1 Transfer approximately 10 g 10 g of sample, weighed to the nearest 0.001 g 0.001 g into a 250-mL250 mL Erlenmeyer
flask, designating this weight as A. Add 50 mL 50 mL of ethyl ether and stir to dissolve the sample. Add 100 mL 100 mL of dilute
HCl (1 + 1) and swirl to mix thoroughly until reaction is complete. In analyzing barium sulfonate products if barium chloride
crystallizes out, add sufficient water to redissolve.
8.1.2 Quantitatively transfer the mixture to a 500-mL500 mL separatory funnel. Shake well, let settle, and draw the aqueous acid
layer into a 250-mL250 mL separatory funnel. Extract the aqueous acid layer in the 250-mL250 mL separatory funnel with three
50-mL50 mL portions of ethyl ether, using the ethyl ether washes to rinse the Erlenmeyer flask first. Combine all the ethyl ether
extracts in the 500-mL500 mL separatory funnel and wash with 50 mL 50 mL of dilute HCl (1 + 3). Combine all the aqueous acid
layers and reextract them with 50 mL 50 mL of ethyl ether.
8.2 Conversion of Sulfonic Acid to Sodium Sulfonate:
8.2.1 Collect all of the ether washes in the 500-mL500 mL separatory funnel and shake with successive 50-mL50 mL portions
of Na SO solution containing 2 to 3 drops of methyl orange indicator until a washing does not appear pink. Discard the salt
2 4
washes.
8.2.2 Drain off as much of the aqueous layer as possible from the washed ether solution. Lay the separatory funnel on its side
and introduce about 10 g 10 g of anhydrous Na SO . Stopper the funnel, making sure that the funnel mouth is free of Na SO
2 4 2 4
crystals and shake the mixture vigorously for 33 min to 4 min, 4 min, to remove the last traces of water, venting the funnel
frequently. Place a 250-mL250 mL Erlenmeyer flask on a steam bath and filter the ether solution through a small plug of cotton,
placed in the vortex of a filter funnel, into the Erlenmeyer flask, keeping approximately 50 mL 50 mL of solution in the Erlenmeyer
flask while evaporating. Rinse the funnel and filter with 50 mL 50 mL of ethyl ether, adding the rinsing to the main ether solution.
Evaporate the ethyl ether until approximately 10 mL 10 mL of solution remains. Add 50 mL of 99 mass % 50 mL of 99 % by mass
isopropyl alcohol, several drops of phenolphthalein indicator solution, and titrate with 0.10.1 mol mol/L ⁄L standard NaOH
solution to the red color change. Place the flask on a steam bath and evaporate to dryness. Dissolve the sodium sulfonate and oil
residue in chloroform; transfer quantitatively into a 100-mL100 mL volumetric flask, adjust to volume, and proceed directly with
The sole source of supply of silica gel, Grade 62, known to the committee at this time is W.R. Grace and Co., Davison Chemical Corp., Baltimore, MD 21203. If you
are aware of alternative suppliers, please provide this information to ASTM International Headquarters. Your comments will receive careful consideration at a meeting of
the responsible technical committee, which you may attend.
D3712 − 05 (2017)
Section 10. The solution may turn acidic on standing in the laboratory. Should this occur, add sufficient 0.1
...









Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.