ASTM D4625-04(2009)
(Test Method)Standard Test Method for Distillate Fuel Storage Stability at 43°C (110°F)
Standard Test Method for Distillate Fuel Storage Stability at 43°C (110°F)
SIGNIFICANCE AND USE
Fuel oxidation and other degradative reactions leading to formation of sediment (and color) are mildly accelerated by the test conditions compared with typical storage conditions. Test results have been shown to predict storage stability more reliably than other more accelerated tests. See Appendix X1 for information on the correlation of test results with actual field storage.
Because the storage periods are long (4 to 24 weeks), the test method is not suitable for quality control testing, but does provide a tool for research on storage properties of fuels.
Because environmental effects and the materials and nature of tank construction affect storage stability, the results obtained by this test are not necessarily the same as those obtained during storage in a specific field storage situation.
SCOPE
1.1 This test method covers a method for evaluating the inherent storage stability of distillate fuels having flash points above 38°C (100°F), by Test Methods D 93, and 90 % distilled points below 340°C (644°F), by Test Method D 86.
Note 1—ASTM specification fuels falling within the scope of this test method are Specification D 396, Grade Nos. 1 and 2; Specification D 975, Grades 1-D and 2-D; and Specification D 2880, Grades 1-GT and 2-GT.
1.2 This test method is not suitable for quality control testing but, rather it is intended for research use to shorten storage time relative to that required at ambient storage temperatures.
1.3 Appendix X1 presents additional information about storage stability and the correlation of Test Method D 4625 results with sediment formation in actual field storage.
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Buy Standard
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: D4625 − 04(Reapproved 2009)
Designation: 378/87
Standard Test Method for
Middle Distillate Fuel Storage Stability at 43°C (110°F)
This standard is issued under the fixed designation D4625; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
This standard has been approved for use by agencies of the Department of Defense.
1. Scope D396 Specification for Fuel Oils
D975 Specification for Diesel Fuel Oils
1.1 This test method covers a method for evaluating the
D1193 Specification for Reagent Water
inherent storage stability of distillate fuels having flash points
D2880 Specification for Gas Turbine Fuel Oils
above 38°C (100°F), by Test Methods D93, and 90 % distilled
D4057 Practice for Manual Sampling of Petroleum and
points below 340°C (644°F), by Test Method D86.
Petroleum Products
NOTE 1—ASTM specification fuels falling within the scope of this test
D4177 Practice for Automatic Sampling of Petroleum and
method are Specification D396, Grade Nos. 1 and 2; Specification D975,
Petroleum Products
Grades 1-D and 2-D; and Specification D2880, Grades 1-GT and 2-GT.
1.2 This test method is not suitable for quality control
3. Terminology
testing but, rather it is intended for research use to shorten
3.1 Definitions of Terms Specific to This Standard:
storage time relative to that required at ambient storage
temperatures. 3.1.1 adherent insolubles, n—gums formed during storage
that remain tightly attached to the walls of the vessel.
1.3 Appendix X1 presents additional information about
storage stability and the correlation of Test Method D4625
3.1.2 filterable insolubles, n—solids formed during storage
results with sediment formation in actual field storage. that can be removed from the fuel by filtration.
1.4 This standard does not purport to address all of the
3.1.3 inherent storage stability, n—of middle distillate
safety concerns, if any, associated with its use. It is the
fuel—theresistancetochangeinstorageincontactwithair,but
responsibility of the user of this standard to establish appro-
in the absence of other environmental factors such as water, or
priate safety and health practices and determine the applica-
reactive metallic surfaces and dirt.
bility of regulatory limitations prior to use.
3.1.4 total insolubles, n—sum of the filterable insolubles
plus the adherent insolubles.
2. Referenced Documents
2.1 ASTM Standards:
4. Summary of Test Method
D86 Test Method for Distillation of Petroleum Products at
Atmospheric Pressure
4.1 Four-hundred millilitre volumes of filtered fuel are aged
D93 Test Methods for Flash Point by Pensky-Martens
by storage in borosilicate glass containers at 43°C (110°F) for
Closed Cup Tester
periods of 0, 4, 8, 12, 18, and 24 weeks. After aging for a
D381 Test Method for Gum Content in Fuels by Jet Evapo-
selected time period, a sample is removed from storage, cooled
ration
to room temperature, and analyzed for filterable insolubles and
for adherent insolubles.
This test method is under the jurisdiction of ASTM Committee D02 on
5. Significance and Use
Petroleum Products and Lubricantsand is the direct responsibility of Subcommittee
D02.14 on Stability and Cleanliness of Liquid Fuels.
5.1 Fuel oxidation and other degradative reactions leading
Current edition approved June 1, 2009. Published August 2009. Originally
to formation of sediment (and color) are mildly accelerated by
approved in 1986. Last previous edition approved in 2004 as D4625–04.
the test conditions compared with typical storage conditions.
This test method was adopted as a joint ASTM/IP standard in 1986. DOI:
10.1520/D4625-04R09.
Test results have been shown to predict storage stability more
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
reliablythanothermoreacceleratedtests.SeeAppendixX1for
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
information on the correlation of test results with actual field
Standards volume information, refer to the standard’s Document Summary page on
the ASTM website. storage.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
D4625 − 04 (2009)
FIG. 1 Schematic of Filtration System
5.2 Becausethestorageperiodsarelong(4to24weeks),the 7. Reagents and Materials
test method is not suitable for quality control testing, but does
7.1 Purity of Reagents—Reagent grade chemicals shall be
provide a tool for research on storage properties of fuels.
used in all tests. Unless otherwise indicated, it is intended that
5.3 Because environmental effects and the materials and all reagents conform to the specifications of the Committee on
nature of tank construction affect storage stability, the results Analytical Reagents of the American Chemical Society where
obtained by this test are not necessarily the same as those such specifications are available. Other grades may be used,
obtained during storage in a specific field storage situation. provided it is first ascertained that the reagent is of sufficiently
high purity to permit its use without lessening the accuracy of
6. Apparatus
the determination.
6.1 Sample Containers, borosilicate glass bottles. The con-
7.2 Nylon Test and Control Membrane Filters—plain,
tainers must have a lid or cover, preferably with a polytetra-
47-mm diameter, nominal pore size 0.8-µm. (Membrane filters
fluoroethylene (PTFE) insert and a hole for a borosilicate glass
with a grid imprinted on their surface may be used as control
vent. The total capacity of the containers is 500 mL.
membrane filters for identification.)
6.2 Storage Oven, large enough to contain all of the sample
7.3 Hydrocarbon Solvent, 2,2,4-trimethylpentane (iso-
bottles. The oven shall be thermostatically controlled to main-
octane)—ASTM knock test reference fuel grade, prefiltered
tain a temperature of 43 6 1°C (110 6 2°F). It shall be as dark
through two glass-fiber filters. (Warning—Extremely flam-
as possible to prevent degradation due to photolytic reactions
mable. Harmful if inhaled. Vapors may cause flash fire.)
and shall also be explosion proof.
7.4 Adherent Insolubles Solvent (Warning—Extremely
6.3 Filter Drying Oven, shall be capable of safely evapo-
flammable. Vapors harmful. May cause flash fire) —Mix equal
rating the solvent at 90 6 5°C for the drying of filter materials.
volumes of reagent grade acetone (Warning— Extremely
flammable. Vapors may cause flash fire), methyl alcohol
6.4 Filtration System—Arrange the following components
(Warning—Flammable. Vapor harmful. May be fatal or cause
as shown in Fig. 1.
blindness if swallowed or inhaled. Cannot be made nonpoison-
6.4.1 Funnel and Funnel Base, with filter support for a
ous), and toluene (Warning—Flammable. Vapor harmful.).
47-mm diameter membrane and a locking ring or spring action
clip.
7.5 Purity of Water—Unless otherwise indicated, references
6.4.2 Ground/Bond Wire, 0.912–2.59 mm (No. 10–No. 19)
to water mean reagent water as defined by Type III of
bare-stranded, flexible stainless steel or copper installed in the
Specification D1193.
flasks and grounded as shown in Fig. 1.
7.6 Liquid or Powder Detergent , water-soluble, for clean-
6.4.3 Receiving Flask, 1.5 L or larger borosilicate glass
ing glassware.
vacuum filter flask, which the filtration apparatus fits into,
equipped with a sidearm to connect to the safety flask.
8. Sampling Procedure
6.4.4 Safety Flask, 1.5 Lor larger borosilicate glass vacuum
8.1 Samples for testing shall be obtained by an appropriate
filter flask equipped with a sidearm to connect the vacuum
method outlined in Practice D4057 or D4177. Sample contain-
system. A fuel and solvent resistance rubber hose, through
ers should be 1 gal (3.78 L) or larger, epoxy-lined cans. Fill
which the grounding wire passes, shall connect the sidearm of
the receiving flask to the tube passing through the rubber
stopper in the top of the safety flask.
Reagent Chemicals, American Chemical Society Specifications, American
Chemical Society, Washington, DC. For Suggestions on the testing of reagents not
6.4.5 Vacuum System, either a water-aspirated or a mechani-
listed by the American Chemical Society, see Annual Standards for Laboratory
cal vacuum pump may be used if capable of producing a
Chemicals, BDH Ltd., Poole, Dorset, U.K., and the United States Pharmacopeia
vacuum of up to 100 kPa below atmospheric pressure when
and National Formulary, U.S. Pharmacopeial Convention, Inc. (USPC), Rockville,
measured at the receiving flask. MD.
D4625 − 04 (2009)
9.3.1 Each set of test filters consists of one test membrane
filter and one control membrane filter. For fuels containing
little particulate materials, only one set of filters is required. If
the fuel is highly contaminated, more than one set of filters
may be required. The two membrane filters used for each
individual test shall be identified by marking the petri dishes
used to hold and transport the filters. Clean all glassware used
in preparation of membrane filters as described in 9.2.
9.3.1.1 Using forceps, place the test and control membrane
filters side by side in a clean petri dish. To facilitate handling,
the membrane filters should rest on clean glass support rods, or
watch glasses, in the petri dish.
9.3.1.2 Place the petri dish, with its lid slightly ajar, in a
drying oven at 90 6 5°C and leave it for 30 min.
9.3.1.3 Remove the petri dish from the drying oven, and
place it near the balance. Keep the petri dish cover ajar, but
keep it such that the membrane filters are still protected from
contamination from the atmosphere. Allow 30 min for the
membrane filters to come to equilibrium with room air tem-
perature and humidity.
9.3.1.4 Remove the control membrane filter from the petri
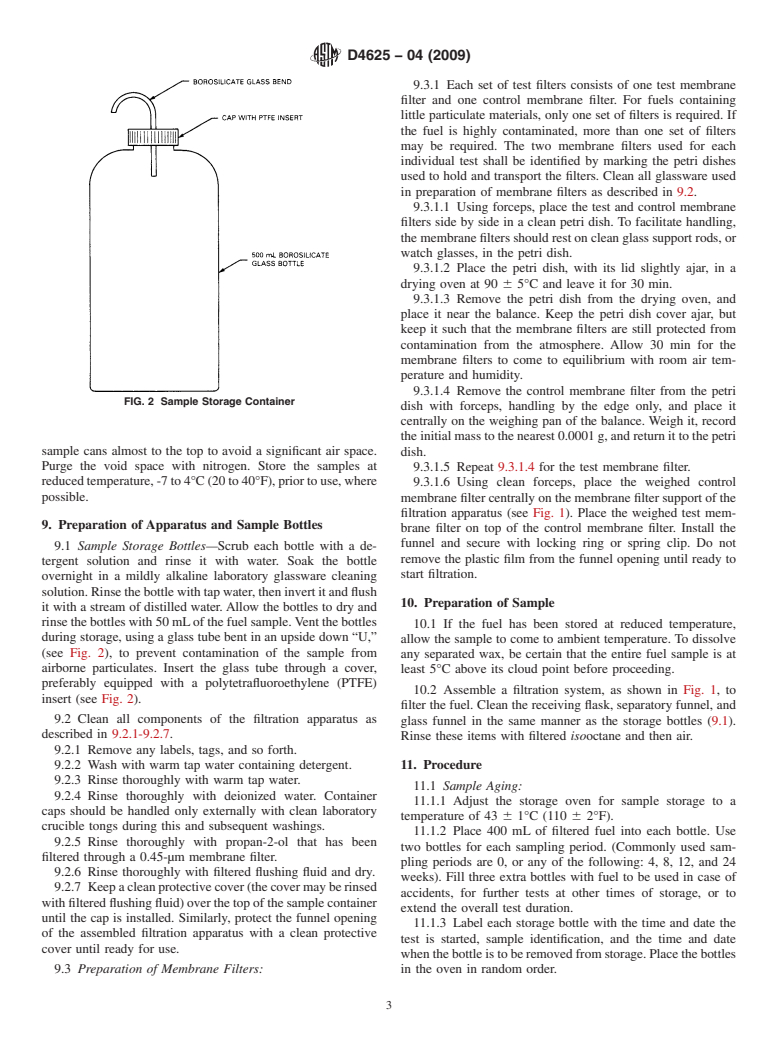
FIG. 2 Sample Storage Container
dish with forceps, handling by the edge only, and place it
centrally on the weighing pan of the balance. Weigh it, record
theinitialmasstothenearest0.0001g,andreturnittothepetri
sample cans almost to the top to avoid a significant air space.
dish.
Purge the void space with nitrogen. Store the samples at
9.3.1.5 Repeat 9.3.1.4 for the test membrane filter.
reducedtemperature,-7to4°C(20to40°F),priortouse,where
9.3.1.6 Using clean forceps, place the weighed control
possible.
membrane filter centrally on the membrane filter support of the
filtration apparatus (see Fig. 1). Place the weighed test mem-
9. Preparation of Apparatus and Sample Bottles
brane filter on top of the control membrane filter. Install the
funnel and secure with locking ring or spring clip. Do not
9.1 Sample Storage Bottles—Scrub each bottle with a de-
remove the plastic film from the funnel opening until ready to
tergent solution and rinse it with water. Soak the bottle
start filtration.
overnight in a mildly alkaline laboratory glassware cleaning
solution.Rinsethebottlewithtapwater,theninvertitandflush
10. Preparation of Sample
it with a stream of distilled water.Allow the bottles to dry and
rinsethebottleswith50mLofthefuelsample.Ventthebottles
10.1 If the fuel has been stored at reduced temperature,
during storage, using a glass tube bent in an upside down “U,”
allow the sample to come to ambient temperature. To dissolve
(see Fig. 2), to prevent contamination of the sample from
any separated wax, be certain that the entire fuel sample is at
airborne particulates. Insert the glass tube through a cover,
least 5°C above its cloud point before proceeding.
preferably equipped with a polytetrafluoroethylene (PTFE)
10.2 Assemble a filtration system, as shown in Fig. 1,to
insert (see Fig. 2).
filter the fuel. Clean the receiving flask, separatory funnel, and
9.2 Clean all components of the filtration apparatus as
glass funnel in the same manner as the storage bottles (9.1).
described in 9.2.1-9.2.7.
Rinse these items with filtered isooctane and then air.
9.2.1 Remove any labels, tags, and so forth.
9.2.2 Wash with warm tap water containing detergent. 11. Procedure
9.2.3 Rinse thoroughly with warm tap water.
11.1 Sample Aging:
9.2.4 Rinse thoroughly with deionized water. Container
11.1.1 Adjust the storage oven for sample storage to a
caps should be handled only externally with clean laboratory
temperature of 43 6 1°C (110 6 2°F).
crucible tongs during this and subsequent washings.
11.1.2 Place 400 mL of filtered fuel into each bottle. Use
9.2.5 Rinse thoroughly with propan-2-ol that has been
two bottles for each sampling period. (Commonly used sam-
filtered through a 0.45-µm membrane filter.
pling periods are 0, or any of the following: 4, 8, 12, and 24
9.2.6 Rinse thoroughly with filtered flushing fluid and dry.
weeks). Fill three extra bottles with fuel to be used in case of
9.2.7 Keepacleanprotectivecover(thecovermayberinsed
accidents, for further tests at other times of storage, or to
withfilteredflushingfluid)overthetopofthesamplecontainer
extend the overall test duration.
until the cap is installed. Similarly, protect the funnel opening
11.1.3 Label each storage bottle with the time and date the
of the assembled filtration apparatus with a clean protective
test is started, sample identification, and the time and date
cover until ready for use.
whenthebottleistoberemovedfromstorage.Placethebottles
9.3 Preparation of Membrane Filters: in the oven in random order.
D4625 − 04 (2009)
11.1.4 Perform zero-week analyses on the same day as the
other samples are placed in storage. Zero-week data are
necessary to provide base data and assure satisfactory tech-
nique.
11.2 Determination of Filterable Insolubles:
11.2.1 At the end of each prescribed period of time, remove
two bottles from the storage oven and allow them to cool to 21
to 27°C (70 to 80°F) in a dark environment. This may take
from4to24h.
11.2.2 After cooling, pour fuel from the sample container to
the graduated cylinder, start the vacuum, and then transfer 100
mL of fuel to the filter funnel.
FIG. 3 Repeatability and Reproducibility for Total Insolubles
11.2.2.1 Continue transferring 100-mLincrements of fuel to
Measurements
the filter funnel. When all the fuel from the sample container
hasbeenfiltered,oriffiltrationslowssothat100mLofsample
requires greater than 10 min for complete filtration, then
desiccator without desiccant and allow to cool to room
remove the filter support/filter funnel from the receiving flask,
temperature. Weigh the beakers to the nearest 0.1 mg. Use a
pourthefilteredfuelintoacleangraduatedcylinder,andrecord
tare beaker (moisture blank) in accordance with Test Method
the volume of fuel that was filtered in millilitres. Keep t
...
This document is not anASTM standard and is intended only to provide the user of anASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately,ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
An American National Standard
Designation:D4625–03 Designation: D 4625 – 04 (Reapproved 2009)
Designation: 378/87
Standard Test Method for
Middle Distillate Fuel Storage Stability at 43°C (110°F)
This standard is issued under the fixed designation D4625; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
This standard has been approved for use by agencies of the Department of Defense.
1. Scope
1.1This test method covers a method for evaluating the inherent storage stability of distillate fuels having flash points above
38°C (100°F) and 90% distilled points below 340°C (644°F).
1.1 This test method covers a method for evaluating the inherent storage stability of distillate fuels having flash points above
38°C (100°F), by Test Methods D93, and 90% distilled points below 340°C (644°F), by Test Method D86.
NOTE 1—ASTM specification fuels falling within the scope of this test method are Specification D396, Grade Nos. 1 and 2; Specification D975,
Grades 1-D and 2-D; and Specification D2880, Grades 1-GT and 2-GT.
1.2 This test method is not suitable for quality control testing but, rather it is intended for research use to shorten storage time
relative to that required at ambient storage temperatures.
1.3 AppendixX1presentsadditionalinformationaboutstoragestabilityandthecorrelationofTestMethodD4625resultswith
sediment formation in actual field storage.
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility
of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory
limitations prior to use.
2. Referenced Documents
2.1 ASTM Standards:
D86 Test Method for Distillation of Petroleum Products at Atmospheric Pressure
D93 Test Methods for Flash Point by Pensky-Martens Closed Cup Tester
D381 Test Method for Existent Gum Content in Fuels by Jet Evaporation
D396 Specification for Fuel Oils
D975 Specification for Diesel Fuel Oils
D1193 Specification for Reagent Water
D2880 Specification for Gas Turbine Fuel Oils
D4057 Practice for Manual Sampling of Petroleum and Petroleum Products Practice for Manual Sampling of Petroleum and
Petroleum Products
D4177 Practice for Automatic Sampling of Petroleum and Petroleum Products
3. Terminology
3.1 Definitions of Terms Specific to This Standard:
3.1.1 adherent insolubles, n—gums formed during storage whichthat remain tightly attached to the walls of the vessel.
3.1.2 filterable insolubles, n—solids formed during storage whichthat can be removed from the fuel by filtration.
3.1.3 inherent storage stability, n—of middle distillate fuel—the resistance to change in storage in contact with air, but in the
absence of other environmental factors such as water, or reactive metallic surfaces and dirt.
This test method is under the jurisdiction ofASTM Committee D02 on Petroleum Products and Lubricants and is the direct responsibility of Subcommittee D02.14 on
Stability and Cleanliness of Liquid Fuels.
Current edition approved Nov.June 1, 2003.2009. Published December 2003.August 2009. Originally approved in 1986. Last previous edition approved in 19982004 as
D4625–92(1998). D4625–04.
This test method was adopted as a joint ASTM/IP standard in 1986.
ForreferencedASTMstandards,visittheASTMwebsite,www.astm.org,orcontactASTMCustomerServiceatservice@astm.org.For Annual Book of ASTM Standards
volume information, refer to the standard’s Document Summary page on the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
D 4625 – 04 (2009)
3.1.4 total insolubles, n—sum of the filterable insolubles plus the adherent insolubles.
4. Summary of Test Method
4.1 Four-hundred millilitre volumes of filtered fuel are aged by storage in borosilicate glass containers at 43°C (110°F) for
periods of 0, 4, 8, 12, 18, and 24 weeks.After aging for a selected time period, a sample is removed from storage, cooled to room
temperature, and analyzed for filterable insolubles and for adherent insolubles.
5. Significance and Use
5.1 Fuel oxidation and other degradative reactions leading to formation of sediment (and color) are mildly accelerated by the
testconditions,comparedtowithtypicalstorageconditions.Testresultshavebeenshowntopredictstoragestabilitymorereliably
than other more accelerated tests. See Appendix X1 for information on the correlation of test results with actual field storage.
5.2 Because the storage periods are long (4 to 24 weeks), the test method is not suitable for quality control testing, but does
provide a tool for research on storage properties of fuels.
5.3 Because environmental effects and the materials and nature of tank construction affect storage stability, the results obtained
by this test are not necessarily the same as those obtained during storage in a specific field storage situation.
6. Apparatus
6.1 Sample Containers, borosilicate glass bottles. The containers must have a lid or cover, preferably with a polytetrafluoro-
ethylene (PTFE) insert and a hole for a borosilicate glass vent. The total capacity of the containers is 500 mL.
6.2 Storage Oven, large enough to contain all of the sample bottles. The oven shall be thermostatically controlled to maintain
atemperatureof43 61°C(110 62°F).Itshallbeasdarkaspossibletopreventdegradationduetophotolyticreactionsandshall
also be explosion proof.
6.3 Filter Drying Oven, shall be capable of safely evaporating the solvent at 90 6 5°C for the drying of filter materials.
6.4 Filtration System—Arrange the following components as shown in Fig. 1.
6.4.1 Funnel and Funnel Base, with filter support for a 47–mm47-mm diameter membrane and a locking ring or spring action
clip.
6.4.2 Ground/Bond Wire,0.912–2.59mm(No.10–No.19)bare-stranded,flexiblestainlesssteelorcopperinstalledintheflasks
and grounded as shown in Fig. 1.
6.4.3 Receiving Flask,1.5Lorlargerborosilicateglassvacuumfilterflask,whichthefiltrationapparatusfitsinto,equippedwith
a sidearm to connect to the safety flask.
6.4.4 Safety Flask,1.5Lorlargerborosilicateglassvacuumfilterflaskequippedwithasidearmtoconnectthevacuumsystem.
Afuelandsolventresistancerubberhose,throughwhichthegroundingwirepasses,shallconnectthesidearmofthereceivingflask
to the tube passing through the rubber stopper in the top of the safety flask.
6.4.5 Vacuum System, either a water-aspirated or a mechanical vacuum pump may be used if capable of producing a vacuum
of 1up to 100 kPa below atmospheric pressure when measured at the receiving flask.
7. Reagents and Materials
7.1 Purity of Reagents—Reagent grade chemicals shall be used in all tests. Unless otherwise indicated, it is intended that all
reagents conform to the specifications of the Committee on Analytical Reagents of the American Chemical Society where such
FIG. 1 Schematic of Filtration System
D 4625 – 04 (2009)
specificationsareavailable. Othergradesmaybeused,provideditisfirstascertainedthatthereagentisofsufficientlyhighpurity
to permit its use without lessening the accuracy of the determination.
7.2 Nylon Test and Control Membrane Filters—plain,47-mmdiameter,nominalporesize0.8-µm.(Membranefilterswithagrid
imprinted on their surface may be used as control membrane filters for identification.)
7.3 Hydrocarbon Solvent, 2,2,4-trimethylpentane (iso-octane)—ASTMknocktestreferencefuelgrade,prefilteredthroughtwo
glass-fiber filters. (Warning—Extremely flammable. Harmful if inhaled. Vapors may cause flash fire.)
7.4 Adherent Insolubles Solvent (Warning—Extremelyflammable.Vaporsharmful.Maycauseflashfire)—Mixequalvolumes
ofreagentgradeacetone(Warning—Extremelyflammable.Vaporsmaycauseflashfire),methylalcohol(Warning—Flammable.
Vapor harmful. May be fatal or cause blindness if swallowed or inhaled. Cannot be made nonpoisonous), and toluene
(Warning—Flammable. Vapor harmful.).
7.5 Purity of Water—Unlessotherwiseindicated,referencestowatermeanreagentwaterasdefinedbyTypeIIIofSpecification
D1193.
7.6 Liquid or Powder Detergent , water-soluble, for cleaning glassware.
8. Sampling Procedure
8.1 Samples for testing shall be obtained by an appropriate method outlined in Practice D4057 or D4177. Sample containers
should be 1 gal (3.78 L) or larger, epoxy-lined cans. Fill sample cans almost to the top to avoid a significant air space. Purge the
void space with nitrogen. Store the samples at reduced temperature, −7-7 to 4°C (20 to 40°F), prior to use, where possible.
9. Preparation of Apparatus and Sample Bottles
9.1 Sample Storage Bottles—Scrub each bottle with a detergent solution and rinse it with water. Soak the bottle overnight in
a mildly alkaline laboratory glassware cleaning solution. Rinse the bottle with tap- water, then invert it and flush it with a stream
ofdistilledwater.Allowthebottlestodryandrinsethebottleswith50mLofthefuelsample.Ventthebottlesduringstorage,using
a glass tube bent in an upside down “U,” (see Fig. 2), to prevent contamination of the sample from airborne particulates. Insert
the glass tube through a cover, preferably equipped with a polytetrafluoroethylene (PTFE) insert (see Fig. 2).
9.2 Clean all components of the filtration apparatus as described in 9.2.1–9.2.79.2.1-9.2.7.
9.2.1 Remove any labels, tags, and so forth.
9.2.2 Wash with warm tap water containing detergent.
9.2.3 Rinse thoroughly with warm tap water.
9.2.4 Rinse thoroughly with deionized water. Container caps should be handled only externally with clean laboratory crucible
tongs during this and subsequent washings.
Reagent Chemicals, American Chemical Society Specifications,American Chemical Society, Washington, DC. For Suggestions on the testing of reagents not listed by
the American Chemical Society, see Annual Standards for Laboratory Chemicals, BDH Ltd., Poole, Dorset, U.K., and the United States Pharmacopeia and National
Formulary, U.S. Pharmacopeial Convention, Inc. (USPC), Rockville, MD.
FIG. 2 Sample Storage Container
D 4625 – 04 (2009)
9.2.5 Rinse thoroughly with propan-2-ol that has been filtered through a 0.45-µm membrane filter.
9.2.6 Rinse thoroughly with filtered flushing fluid and dry.
9.2.7Keep9.2.7 Keep a clean protective cover (the cover may be rinsed with filtered flushing fluid) over the top of the sample
containeruntilthecapisinstalled.Similarly,protectthefunnelopeningoftheassembledfiltrationapparatuswithacleanprotective
cover until ready for use.
9.3 Preparation of Membrane Filters:
9.3.1 Each set of test filters consists of one test membrane filter and one control membrane filter. For fuels containing little
particulate materials, only one set of filters is required. If the fuel is highly contaminated, more than one set of filters may be
required. The two membrane filters used for each individual test shall be identified by marking the petri dishes used to hold and
transport the filters. Clean all glassware used in preparation of membrane filters as described in 9.2.
9.3.1.1 Using forceps, place the test and control membrane filters side by side in a clean petri dish. To facilitate handling, the
membrane filters should rest on clean glass support rods, or watch glasses, in the petri dish.
9.3.1.2 Place the petri dish, with its lid slightly ajar, in a drying oven at 90 6 5°C and leave it for 30 min.
9.3.1.3 Remove the petri dish from the drying oven, and place it near the balance. Keep the petri dish cover ajar, but keep it
such that the membrane filters are still protected from contamination from the atmosphere.Allow 30 min for the membrane filters
to come to equilibrium with room air temperature and humidity.
9.3.1.4 Remove the control membrane filter from the petri dish with forceps, handling by the edge only, and place it centrally
on the weighing pan of the balance. Weigh it, record the initial mass to the nearest 0.0001 g, and return it to the petri dish.
9.3.1.5 Repeat 9.3.1.4 for the test membrane filter.
9.3.1.6 Usingcleanforceps,placetheweighedcontrolmembranefiltercentrallyonthemembranefiltersupportofthefiltration
apparatus (see Fig. 1). Place the weighed test membrane filter on top of the control membrane filter. Install the funnel and secure
with locking ring or spring clip. Do not remove the plastic film from the funnel opening until ready to start filtration.
10. Preparation of Sample
10.1 If the fuel has been stored at reduced temperature, allow the sample to come to ambient temperature. To dissolve any
separated wax, be certain that the entire fuel sample is at least 5°C above its cloud point before proceeding.
10.2 Assemble a filtration system, as shown in Fig. 1, to filter the fuel. Clean the receiving flask, separatory funnel, and glass
funnel in the same manner as the storage bottles (9.1). Rinse these items with filtered isooctane and then air.
11. Procedure
11.1 Sample Aging:
11.1.1 Adjust the storage oven for sample storage to a temperature of 43 6 1°C (110 6 2°F).
11.1.2 Place 400 mL of filtered fuel into each bottle. Use two bottles for each sampling period. (Commonly used sampling
periods are 0, or any of the following: 4, 8, 12, and 24 weeks). Fill three extra bottles with fuel to be used in case of accidents,
for further tests at other times of storage, or to extend the overall test duration.
11.1.3 Label each storage bottle with the time and date the test is started, sample identification, and the time and date when the
bottle is to be removed from storage. Place the bottles in the oven in random order.
11.1.4 Perform zero-week analyses on the same day as the other samples are placed in storage. Zero-week data are necessary
to provide base data and assure satisfactory technique.
11.2 Determination of Filterable Insolubles:
11.2.1 At the end of each prescribed period of time, remove two bottles from the storage oven and allow them to cool to 21
to 27°C (70 to 80°F) in a dark environment. This may take from 4 to 24 h.
11.2.2 Aftercooling,pourfuelfromthesamplecontainertothegraduatedcylinder,startthevacuum,andthentransfer100mL
of fuel to the filter funnel.
11.2.2.1 Continue transferring 100-mLincrements of fuel to the filter funnel. When all the fuel from the sample container has
been filtered, or if filtration slows so that 100 mLof sample requires greater than 10 min for complete filtr
...








Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.