ASTM D1946-90(2000)
(Practice)Standard Practice for Analysis of Reformed Gas by Gas Chromatography
Standard Practice for Analysis of Reformed Gas by Gas Chromatography
SCOPE
1.1 This practice covers the determination of the chemical composition of reformed gases and similar gaseous mixtures containing the following components: hydrogen, oxygen, nitrogen, carbon monoxide, carbon dioxide, methane, ethane, and ethylene.
1.2 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation:D1946–90(Reapproved 2000)
Standard Practice for
Analysis of Reformed Gas by Gas Chromatography
This standard is issued under the fixed designation D 1946; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope 5. Apparatus
1.1 This practice covers the determination of the chemical 5.1 Detector—The detector shall be a thermal conductivity
composition of reformed gases and similar gaseous mixtures type or its equivalent in stability and sensitivity. The thermal
containing the following components: hydrogen, oxygen, ni- conductivity detector must be sufficiently sensitive to produce
trogen, carbon monoxide, carbon dioxide, methane, ethane, a signal of at least 0.5 mV for 1 mol % methane in a 0.5-mL
and ethylene. sample.
1.2 This standard does not purport to address all of the 5.2 Recording Instruments—Either strip chart recorders or
safety concerns, if any, associated with its use. It is the electronic integrators, or both, are used to display the separated
responsibility of the user of this standard to establish appro- components. Although a strip chart recorder is not required
priate safety and health practices and determine the applica- when using electronic integration, it is highly desirable for
bility of regulatory limitations prior to use. evaluation of instrument performance.
5.2.1 Therecorder,whenused,shallbeastripchartrecorder
2. Referenced Documents
with a full-range scale of 5 mV or less (1 mV preferred). The
2.1 ASTM Standards: width of the chart shall be not less than 150 mm. A maximum
E 260 Practice for Packed Column Gas Chromatography
pen response time of2s(1s preferred) and a minimum chart
speed of 10 mm/min shall be required. Faster speeds up to 100
3. Summary of Practice
mm/min are desirable if the chromatogram is to be interpreted
3.1 Components in a sample of reformed gas are physically
using manual methods to obtain areas.
separatedbygaschromatographyandcomparedtocorrespond-
5.2.2 Electronic or Computing Integrators—Proof of sepa-
ing components of a reference standard separated under
ration and response equivalent to that for the recorder is
identical operating conditions, using a reference standard
required for displays other than by chart recorder.
mixture of known composition. The composition of the re-
5.3 Attenuator—If manual methods are used to interpret the
formed gas is calculated by comparison of either the peak
chromatogram, an attenuator must be used with the detector
height or area response of each component with the corre-
output signal to keep the peak maxima within the range of the
sponding value of that component in the reference standard.
recorder chart.The attenuator must be accurate to within 0.5 %
between the attenuator range steps.
4. Significance and Use
5.4 Sample Inlet System:
4.1 The information about the chemical composition can be
5.4.1 The sample inlet system must be constructed of
used to calculate physical properties of the gas, such as heating
materials that are inert and nonadsorptive with respect to the
(calorific) value and relative density. Combustion characteris-
components in the sample. The preferred material of construc-
tics, products of combustion, toxicity, and interchangeability
tion is stainless steel. Copper and copper-bearing alloys are
with other fuel gases may also be inferred from the chemical
unacceptable.
composition.
5.4.2 Provision must be made to introduce into the carrier
gas ahead of the analyzing column a gas-phase sample that has
beenentrappedineitherafixedvolumelooportubularsection.
This practice is under the jurisdiction of ASTM Committee D-3 on Gaseous
The injected volume must be reproducible such that successive
Fuels and is the direct responsibility of Subcommittee D03.07 on Analysis of
runs of the same sample agree within the limits of repeatability
Chemical Composition of Gaseous Fuels.
Current edition approved March 30, 1990. Published May 1990. Originally
for the concentration range as specified in 11.1.1.
published as 1946 – 62 T. Last previous edition D 1946 – 82.
Annual Book of ASTM Standards, Vol 14.02.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
D1946–90 (2000)
5.4.3 If the instrument is calibrated with pure components, 5.8.2 Either an adsorption-type column or a partition-type
the inlet system shall be equipped to introduce a sample at less column, or both, may be used to make the analysis.
than atmospheric pressure. The pressure-sensing device must
NOTE 1—See Practice E 260 for general gas chromatography proce-
be accurate to 0.1 kPa (1 mm Hg).
dures.
5.5 Column Temperature Control:
5.5.1 Isothermal—When isothermal operation is used, the 5.8.2.1 Adsorption Column—This column must completely
analytical columns shall be maintained at a temperature con-
separate hydrogen, oxygen, nitrogen, methane, and carbon
stant to 0.3°C during the course of the sample run and the monoxide. If a recorder is used, the recorder pen must return to
corresponding reference run.
thebaselinebetweeneachsuccessivepeak.Equivalentproofof
5.5.2 Temperature Programming—Temperature program-
separation is required for displays other than by chart recorder.
ming may be used, as feasible. The oven temperature shall not
Fig. 1 is an example chromatogram obtained with an adsorp-
exceed the recommended temperature limit for the materials in
tion column.
the column.
(1) Because of similarities in thermal conductivities, he-
5.6 Detector Temperature Control—The detector tempera-
lium should not be used as the carrier gas for hydrogen when
ture shall be maintained at a temperature constant to 0.3°C
hydrogen is less than 1 % of the sample. Either argon or
during the course of the sample run and the corresponding
nitrogen carrier gas is suitable for both percent and parts per
reference run. The detector temperature shall be equal to, or
million quantities of hydrogen.
greater than, the maximum column temperature.
(2) The use of a carrier gas mixture of 8.5 % hydrogen and
5.7 Carrier Gas—The instrument shall be equipped with
91.5 % helium will avoid the problem of reversing polarities of
suitable facilities to provide flow of carrier gas through the
hydrogen responses as the concentration of hydrogen in the
analyzer and detector at a flow rate that is constant to 1 %
sample is increased.
throughout the analysis of the sample and the reference
(3) The precision of measurement of hydrogen can be
standard. The purity of the carrier gas may be improved by
increased by using a separate injection for hydrogen, using
flowing the carrier gas through selective filters before its entry
either argon or nitrogen for the carrier gas.
into the chromatograph.
5.8 Columns: (4) Another technique for isolating the hydrogen in a
5.8.1 The columns shall be constructed of materials that are sample is to use a palladium transfer tube at the end of the
inert and nonadsorptive with respect to the components in the adsorption column; this will permit only hydrogen to be
sample. The preferred material of construction is stainless transferred to a stream of argon or nitrogen carrier gas for
steel. Copper and copper-bearing alloys are unacceptable. analysis in a second thermal conductivity detector.
Column: 2-m by 6-mm inside diameter Type 133 Flow rate: 60-mL helium/min
molecular sieves, 14 to 30 mesh Sample size: 0.5 mL
Temperature: 35°C
FIG. 1 Chromatogram of Reformed Gas on Molecular Sieve Column
D1946–90 (2000)
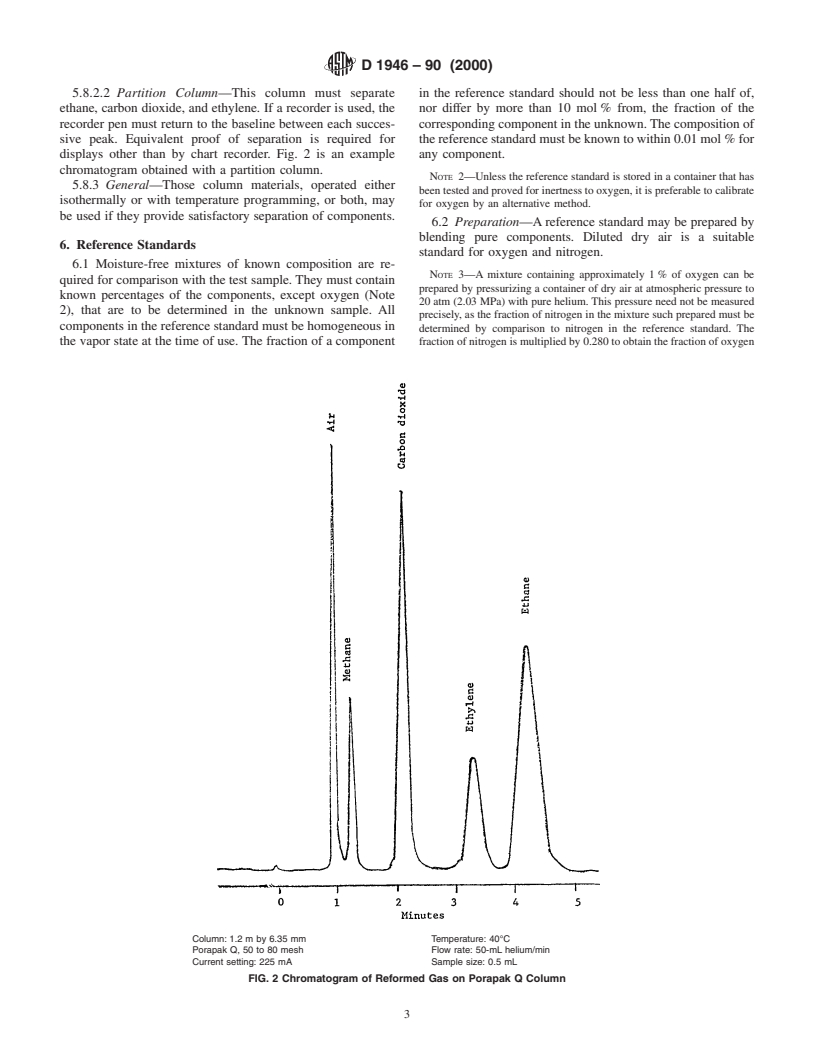
5.8.2.2 Partition Column—This column must separate in the reference standard should not be less than one half of,
ethane, carbon dioxide, and ethylene. If a recorder is used, the nor differ by more than 10 mol % from, the fraction of the
recorder pen must return to the baseline between each succes- corresponding component in the unknown.The composition of
sive peak. Equivalent proof of separation is required for thereferencestandardmustbeknowntowithin0.01mol %for
displays other than by chart recorder. Fig. 2 is an example any component.
chromatogram obtained with a partition column.
NOTE 2—Unless the reference standard is stored in a container that has
5.8.3 General—Those column materials, operated either
been tested and proved for inertness to oxygen, it is preferable to calibrate
isothermally or with temperature programming, or both, may
for oxygen by an alternative method.
be used if they provide satisfactory separation of components.
6.2 Preparation—Areference standard may be prepared by
blending pure components. Diluted dry air is a suitable
6. Reference Standards
standard for oxygen and nitrogen.
6.1 Moisture-free mixtures of known composition are re-
NOTE 3—A mixture containing approximately 1 % of oxygen can be
quired for comparison with the test sample. They must contain
prepared by pressurizing a container of dry air at atmospheric pressure to
known percentages of the components, except oxygen (Note
20 atm (2.03 MPa) with pure helium. This pressure need not be measured
2), that are to be determined in the unknown sample. All
precisely, as the fraction of nitrogen in the mixture such prepared must be
components in the reference standard must be homogeneous in
determined by comparison to nitrogen in the reference standard. The
the vapor state at
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.