ASTM D3339-95(2000)e1
(Test Method)Standard Test Method for Acid Number of Petroleum Products by Semi-Micro Color Indicator Titration
Standard Test Method for Acid Number of Petroleum Products by Semi-Micro Color Indicator Titration
SCOPE
1.1 This test method covers the determination of acidic constituents in new or used petroleum products and lubricants soluble or nearly soluble in mixtures of toluene, and isopropyl alcohol. The test method is especially intended for cases in which the amount of sample available to be analyzed is too small to allow accurate analysis by Test Methods D974 or D664. It is applicable for the determination of acids having dissociation constants in water larger than 10-9. Extremely weak acids having dissociation constants smaller than 10-9 do not interfere. Salts titrate if their hydrolysis constants are larger than 10-9.
1.2 This test method can be used to indicate relative changes in acid number that occur in an oil during use under oxidizing conditions. Although the titration is made under definite equilibrium conditions, the method does not measure an absolute acidic property that can be used to predict performance of an oil under service conditions. No general relationship between bearing corrosion and acid number is known.
1.3 Since this test method requires substantially less sample than Test Methods D974 or D664, it provides an advantageous means of monitoring an oxidation test by changes in acid number by (a) minimizing test sample depletion for acid number analyses and thus minimizing the disturbance of the test or (b) allowing additional acid number analyses to be made while maintaining the same test sample depletion and thus providing additional data.
Note 1--Some oils, such as many cutting oils, rust-proofing oils, and similar compounded oils, or excessively dark-colored oils, may be more difficult to analyze by this test method due to obscurity of the color-indicator end point. These oils can be analyzed by Test Method D664 provided sufficient sample is available. However, this situation is much less likely using Test Method D3339 than using Test Method D974 due to the use of a more highly dilute sample during the titration and due to the greater stability of the end point color change. The acid numbers obtained by Test Method D3339 may or may not be numerically the same as those obtained by Test Method D664 but they should be of the same order of magnitude.
Note 2--The results obtained using this method have been found to be numerically the same as those obtained using Test Method D974, within the precision of the two methods, for new or oxidized lubricants of the type primarily intended for hydraulic or steam turbine type service. The oxidized lubricants were obtained using the Test Method D943 oxidation test. This correlation is shown by the correlation coefficient r = 0.989 with slope s = + 1.017 and intercept y = + 0.029, calculated using the acid numbers obtained using both titration methods for the samples used for the precision statement (12.2).
1.4 The values stated in acceptable SI units are to be regarded as the standard. Values in inch-pound units are for information only.
1.5 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use. For specific hazard statements, see Sections 7 and 9 and A1.14 and A2.3.1.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superceded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
e1
Designation: D 3339 – 95 (Reapproved 2000) An American National Standard
Standard Test Method for
Acid Number of Petroleum Products by Semi-Micro Color
Indicator Titration
This standard is issued under the fixed designation D 3339; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
e NOTE—The warning notes were inserted in the text editorially in December 2000.
NOTE 2—The results obtained using this method have been found to be
1. Scope
numerically the same as those obtained using Test Method D 974, within
1.1 This test method covers the determination of acidic
the precision of the two methods, for new or oxidized lubricants of the
constituents in new or used petroleum products and lubricants
type primarily intended for hydraulic or steam turbine type service. The
soluble or nearly soluble in mixtures of toluene, and isopropyl
oxidized lubricants were obtained using the Test Method D 943 oxidation
alcohol. The test method is especially intended for cases in test. This correlation is shown by the correlation coefficient r = 0.989 with
slope s = + 1.017 and intercept y = + 0.029, calculated using the acid
which the amount of sample available to be analyzed is too
numbers obtained using both titration methods for the samples used for the
small to allow accurate analysis by Test Methods D 974 or
precision statement (12.2).
D 664. It is applicable for the determination of acids having
−9
1.4 The values stated in acceptable SI units are to be
dissociation constants in water larger than 10 . Extremely
−9
regarded as the standard. Values in inch-pound units are for
weak acids having dissociation constants smaller than 10 do
information only.
not interfere. Salts titrate if their hydrolysis constants are larger
−9
1.5 This standard does not purport to address all of the
than 10 .
safety concerns, if any, associated with its use. It is the
1.2 This test method can be used to indicate relative changes
responsibility of the user of this standard to establish appro-
in acid number that occur in an oil during use under oxidizing
priate safety and health practices and determine the applica-
conditions. Although the titration is made under definite
bility of regulatory limitations prior to use. For specific hazard
equilibrium conditions, the method does not measure an
statements, see Sections 7 and 9 and A1.1.4 and A2.3.1.
absolute acidic property that can be used to predict perfor-
mance of an oil under service conditions. No general relation-
2. Referenced Documents
ship between bearing corrosion and acid number is known.
2.1 ASTM Standards:
1.3 Since this test method requires substantially less sample
D 664 Test Method for Acid Number of Petroleum Products
than Test Methods D 974 or D 664, it provides an advanta-
by Potentiometric Titration
geous means of monitoring an oxidation test by changes in acid
D 943 Test Method for Oxidation Characteristics of Inhib-
number by (a) minimizing test sample depletion for acid
ited Mineral Oils
number analyses and thus minimizing the disturbance of the
D 974 Test Method for Acid and Base Number by Color-
test or (b) allowing additional acid number analyses to be made
Indicator Titration
while maintaining the same test sample depletion and thus
D 1193 Specification for Reagent Water
providing additional data.
D 4175 Terminology Relating to Petroleum, Petroleum
NOTE 1—Some oils, such as many cutting oils, rust-proofing oils, and
Products, and Lubricants
similar compounded oils, or excessively dark-colored oils, may be more
difficult to analyze by this test method due to obscurity of the color-
3. Terminology
indicator end point. These oils can be analyzed by Test Method D 664
3.1 Definitions:
provided sufficient sample is available. However, this situation is much
less likely using Test Method D 3339 than using Test Method D 974 due
3.1.1 acid number, n—the quantity of base, expressed in
to the use of a more highly dilute sample during the titration and due to
milligrams of potassium hydroxide per gram of sample, that is
the greater stability of the end point color change. The acid numbers
required to titrate a sample to a specified end point.
obtained by Test Method D 3339 may or may not be numerically the same
3.1.1.1 Discussion—In this test method, acids or salts with
as those obtained by Test Method D 664 but they should be of the same
order of magnitude.
Use of the correlation coefficient is given in Mack, C., Essentials of Statistics
This test method is under the jurisdiction of ASTM Committee D02 on for Scientists and Technologists, Plenum Press, New York, NY, 1967, or other
Petroleum Products and Lubricantsand is the direct responsibility of Subcommittee publications on statistics.
D02.06 on Analysis of Lubricants. Annual Book of ASTM Standards, Vol 05.01.
Current edition approved Aug. 15, 1995. Published October 1995. Originally Annual Book of ASTM Standards, Vol 11.01.
published as D 3339 – 74. Last previous edition D 3339 – 92. Annual Book of ASTM Standards, Vol 05.02.
Copyright © ASTM, 100 Barr Harbor Drive, West Conshohocken, PA 19428-2959, United States.
NOTICE: This standard has either been superceded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
D 3339
−9
dissociation constants greater than 10 , are titrated to a green mensions are 53 mm top diameter, 45 mm bottom diameter,
end point with p-naphtholbenzein indicator. and 25 mm height. The stopper is fitted with a 8-mm (0.3-in.)
3.1.2 used oil, n—any oil that has been in a piece of outside diameter glass inlet tube extending 15 6 2 mm beyond
equipment (for example, an engine, gearbox, transformer or the bottom of the stopper and with a 7 6 1-mm inside diameter
turbine) whether operated or not. hole. The inlet tube and hole are placed on opposite sides of the
3.1.2.1 Discussion—Typically, in this test method, the acid- stopper with a center-to-center separation distance of 30 6 1
ity of oxidized hydraulic or steam turbine oils is measured. mm.
D 4175 6.5 Purge Gas Rotameter, capable of indicating a flow rate
of 10 L/h.
4. Summary of Test Method
6.6 Stirrer Motor, variable speed, magnetically linked.
4.1 To determine the acid number a sample of the oil is
6.7 Stirring Bar, cylindrical, TFE-fluorocarbon encased,
dissolved in a solvent consisting of toluene, isopropyl alcohol, 25.4 mm long and 7.9 mm in diameter.
and a small amount of water. The resulting single-phase
6.8 Pipet, capable of transferring 0.100 6 0.002 mL of
solution is titrated at room temperature under a nitrogen titration indicator solution.
atmosphere with standardized 0.01 M potassium hydroxide
6.9 Titration Solvent Buret—A 500-mL or larger capacity
(KOH) in isopropyl alcohol to the stable green color of the buret with 5-mL subdivisions. The top of the buret is stoppered
added p-naphtholbenzein indicator.
and connected with an absorption tube, as in 6.2, to remove
atmospheric carbon dioxide and water. An alternative means of
5. Significance and Use
dispensing the titration solvent may be used provided a
5.1 This test method measures the acid number of oils
dispensing repeatability within 6 1 mL for 40 mL is obtainable
obtained from laboratory oxidation tests using smaller amounts
and the solvent in the dispenser is isolated from atmospheric
of sample than Test Methods D 974 or D 664. It has specific
carbon dioxide and water.
application in Test Method D 943 in which small aliquots of oil
7. Reagents and Materials
are periodically removed for testing by Test Method D 3339.
This test method, therefore, provides a means of monitoring the
7.1 Purity of Reagents—Reagent grade chemicals shall be
relative oxidation of oils, by measuring changes in acid used in all tests. Unless otherwise indicated, it is intended that
number, at different time intervals and under the various
all reagents shall conform to the specifications of the Commit-
oxidizing test conditions. tee on Analytical Reagents of the American Chemical Society,
where such specifications are available or to other such
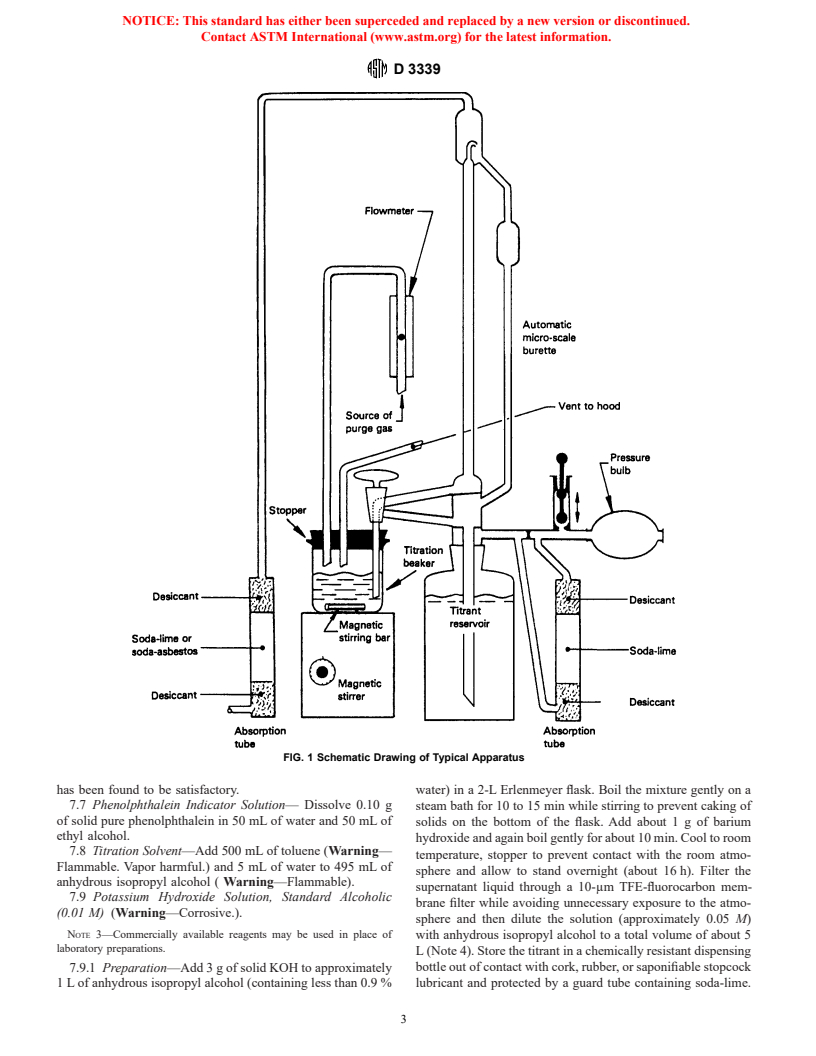
6. Apparatus (Refer to Fig. 1) 6
recognized standards for reagent chemicals. Other grades may
6.1 Titration Buret—A micro scale, automatic buret with
be used, provided it is first ascertained that the reagent is of
0.01 mL subdivisions and at least a 2-mL buret capacity.
sufficient high purity to permit its use without lessening the
6.2 Titrant Reservoir—The preferred reservoir is one that is
accuracy of the determination.
integral with the automatic buret, such as shown in Fig. 1. A
7.2 Purity of Water—All references to water shall be
titrant reservoir separate from the automatic buret may be used
understood to mean freshly distilled (carbon dioxide-free)
if the line connecting the reservoir with the buret is all glass.
water conforming to Specification D 1193, Type II or III.
Exposure of the titrant to light should be minimized by use of
7.3 Ethyl Alcohol (Warning—Flammable. Denatured—
amber glass for the reservoir, by wrapping the reservoir with
Cannot be made nontoxic.)—USP 200 proof or denatured
foil such as aluminum foil, or by other suitable means. Also,
alcohol according to Formula 30 of the U. S. Bureau of Internal
the tube in the reservoir for titrant withdrawal is adjusted so
Revenue.
that the end of the tube is about 20 mm (0.8 in.) from the
7.4 Isopropyl Alcohol, (2-Propanol) Anhydrous,
bottom of the reservoir so that any precipitate that may collect
(Warning—Flammable.) containing less than 0.9 % water.
on the bottom of the reservoir will not be disturbed. To further
7.5 p-Naphtholbenzein Indicator Solution—The
avoid disturbing any precipitate in the reservoir, movement of
p-naphtholbenzein must meet the specifications given in the
the reservoir must be minimized.
Annex. Prepare a solution containing 10 g of
6.2.1 With either type of reservoir all entrances and exits to
p-naphtholbenzein per litre of titration solvent.
the buret and reservoir must be connected to absorption tubes
7.6 Nitrogen, dry and carbon dioxide-free.
to remove atmospheric carbon dioxide and water, for example,
7.6.1 In order to obtain repeatable results and a stable end
tubes containing 10 to 20-mesh anhydrous calcium sulfate as
point color change, it is especially important that the nitrogen
desiccant and soda-lime as carbon dioxide absorbent. Precau-
purge gas be free of carbon dioxide. Prepurified grade nitrogen
tions must be taken to prevent introduction of any soda-lime
into the titrant reservoir or buret.
Reagent Chemicals, American Chemical Society Specifications, American
6.3 Titration Beaker—100-mL capacity tall-form Berzelius
Chemical Society, Washington, DC. For suggestions on the testing of reagents not
beaker without pouring spout. Approximate dimensions are 47
listed by the American Chemical Society, see Analar Standards for Laboratory
mm in inside diameter and 79 mm in height.
Chemicals, BDH Ltd., Poole, Dorset, U.K., and the United States Pharmacopeia
and National Formulary, U.S. Pharmacopeial Convention, Inc. (USPC), Rockville,
6.4 Titration Beaker Purging Stopper— A stopper to en-
MD.
close the titration beaker. The stopper must be composed of an
In a 1992 study, only Kodak and Fisher p-naphtholbenzein were found to meet
elastomeric material, such as neoprene, that is essentially
the specifications in Annex A1. The Fisher Reagent solution was the only
unaffected by the titration solvent. Approximate stopper di- commercially available solution to meet the specifications.
NOTICE: This standard has either been superceded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
D 3339
FIG. 1 Schematic Drawing of Typical Apparatus
has been found to be satisfactory. water) in a 2-L Erlenmeyer flask. Boil the mixture gently on a
7.7 Phenolphthalein Indicator Solution— Dissolve 0.10 g steam bath for 10 to 15 min while stirring to prevent caking of
of solid pure phenolphthalein in 50 mL of water and 50 mL of
solids on the bottom of the flask. Add about1gof barium
ethyl alcohol.
hydroxide and again boil gently for about 10 min. Cool to room
7.8 Titration Solvent—Add 500 mL of toluene (Warning—
temperature, stopper to prevent contact with the room atmo-
Flammable. Vapor harmful.) and 5 mL of water to 495 mL of
sphere and allow to stand overnight (about 16 h). Filter the
anhydrous isopropyl alcohol ( Warning—Flammable).
supernatant liquid through a 10-μm TFE-fluorocarbon mem-
7.9 Potassium Hydroxide Solution, Standard Alcoholic
brane filter while avoiding unnecessary exposure to the atmo-
(0.01 M) (Warning—Corrosive.).
sphere and then dilute the solution (approximately 0.05 M)
NOTE 3—Commercially available reagents may be used in place of
with anhydrous isopropyl alcohol to a total volume of about 5
laboratory preparations.
L (Note 4). Store the titrant in a chemically resistant dispensing
7.9.1 Preparation—Add3gof solid KOH to approximately bottle out of contact with cork, rubber, or saponifiable stopcock
1 L of anhydrous isopropyl alcohol (containing less than 0.9 % lubricant and protected by a guard tube containing soda-lime.
NOTICE: This standard has either been superceded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
D 3339
Minimize exposure of the titrant to light by storing in the dark sample. Transfer all traces of sediment from the original
or in an amber bottle or by wrapping the bottle with aluminum container to the bottle by violent agitation of portions of the
foil. sample in the original container. After complete suspension of
all sediment, strain the sample or a convenient aliquot of the
NOTE 4—Care should be taken to ensure a final normality of 0.011 6
sample through a 100-mesh screen for the removal of large
0.002.
contaminating particles (Note 8).
NOTE 5—Because of the relatively large coefficient of cubic expansion
of organic liquids, such as isopropyl alcohol, the standard alcoholic
NOTE 6—When samples are visibly free of sediment, the heating
solutions should be standardized at temperatures close to those employed
procedure may be omitted.
in the titrations of samples.
NOTE 7—As used oil can change appreciably in storage, samples should
7.9.2 Standardization— The titrant is standardized (Note 5)
be tested as soon as possible after removal from the lubricating or test
against dr
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.