ASTM G31-72(1999)
(Practice)Standard Practice for Laboratory Immersion Corrosion Testing of Metals
Standard Practice for Laboratory Immersion Corrosion Testing of Metals
SCOPE
1.1 This practice describes accepted procedures for and factors that influence laboratory immersion corrosion tests, particularly mass loss tests. These factors include specimen preparation, apparatus, test conditions, methods of cleaning specimens, evaluation of results, and calculation and reporting of corrosion rates. This practice also emphasizes the importance of recording all pertinent data and provides a checklist for reporting test data. Other ASTM procedures for laboratory corrosion tests are tabulated in the Appendix. Note 1-In many cases the corrosion product on the reactive metals titanium and zirconium is a hard and tightly bonded oxide that defies removal by chemical or ordinary mechanical means. In many such cases, corrosion rates are established by mass gain rather than mass loss.
1.2 The values stated in SI units are to be regarded as the standard. The values given in parentheses are for information only.
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: G 31 – 72 (Reapproved 1999)
Standard Practice for
Laboratory Immersion Corrosion Testing of Metals
This standard is issued under the fixed designation G 31; the number immediately following the designation indicates the year of original
adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A superscript
epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope G 46 Guide for Examination and Evaluation of Pitting
Corrosion
1.1 This practice describes accepted procedures for and
factors that influence laboratory immersion corrosion tests,
3. Significance and Use
particularly mass loss tests. These factors include specimen
3.1 Corrosion testing by its very nature precludes complete
preparation, apparatus, test conditions, methods of cleaning
standardization. This practice, rather than a standardized pro-
specimens, evaluation of results, and calculation and reporting
cedure, is presented as a guide so that some of the pitfalls of
of corrosion rates. This practice also emphasizes the impor-
such testing may be avoided.
tance of recording all pertinent data and provides a checklist
3.2 Experience has shown that all metals and alloys do not
for reporting test data. Other ASTM procedures for laboratory
respond alike to the many factors that affect corrosion and that
corrosion tests are tabulated in the Appendix.
“accelerated” corrosion tests give indicative results only, or
NOTE 1—Warning: In many cases the corrosion product on the reac-
may even be entirely misleading. It is impractical to propose an
tive metals titanium and zirconium is a hard and tightly bonded oxide that
inflexible standard laboratory corrosion testing procedure for
defies removal by chemical or ordinary mechanical means. In many such
general use, except for material qualification tests where
cases, corrosion rates are established by mass gain rather than mass loss.
standardization is obviously required.
1.2 The values stated in SI units are to be regarded as the
3.3 In designing any corrosion test, consideration must be
standard. The values given in parentheses are for information
given to the various factors discussed in this practice, because
only.
these factors have been found to affect greatly the results
1.3 This standard does not purport to address all of the
obtained.
safety concerns, if any, associated with its use. It is the
responsibility of the user of this standard to establish appro-
4. Interferences
priate safety and health practices and determine the applica-
4.1 The methods and procedures described herein represent
bility of regulatory limitations prior to use.
the best current practices for conducting laboratory corrosion
tests as developed by corrosion specialists in the process
2. Referenced Documents
industries. For proper interpretation of the results obtained, the
2.1 ASTM Standards:
specific influence of certain variables must be considered.
A 262 Practices for Detecting Susceptibility to Intergranu-
These include:
lar Attack in Austenitic Stainless Steels
4.1.1 Metal specimens immersed in a specific hot liquid
E 8 Test Methods for Tension Testing of Metallic Materials
may not corrode at the same rate or in the same manner as in
G 1 Practice for Preparing, Cleaning, and Evaluating Cor-
equipment where the metal acts as a heat transfer medium in
rosion Test Specimens
heating or cooling the liquid. If the influence of heat transfer
G 4 Guide for Conducting Corrosion Coupon Tests in Field
effects is specifically of interest, specialized procedures (in
Applications
which the corrosion specimen serves as a heat transfer agent)
G 16 Guide for Applying Statistics to Analysis of Corrosion 6
must be employed (1).
Data
4.1.2 In laboratory tests, the velocity of the environment
relative to the specimens will normally be determined by
convection currents or the effects induced by aeration or
This practice is under the jurisdiction of ASTM Committee G-1 on Corrosion
boiling or both. If the specific effects of high velocity are to be
of Metalsand is the direct responsibility of Subcommittee G01.05 on Laboratory
studied, special techniques must be employed to transfer the
Corrosion Tests.
environment through tubular specimens or to move it rapidly
Current edition approved May 30, 1972. Published July 1972.
past the plane face of a corrosion coupon (2). Alternatively, the
This practice is based upon NACE Standard TM-01-69, “Test Method-
Laboratory Corrosion Testing of Metals for the Process Industries”, with modifica-
tions to relate more directly to Practices G 1 and G 31 and Guide G 4.
Annual Book of ASTM Standards, Vol 01.03.
4 6
Annual Book of ASTM Standards, Vol 03.01. The boldface numbers in parentheses refer to the list of references at the end of
Annual Book of ASTM Standards, Vol 03.02. this practice.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
G31
coupon may be rotated through the environment, although it is Close attention and a more sophisticated evaluation than a
then difficult to evaluate the velocity quantitatively because of simple mass loss measurement are required to detect this
the stirring effects incurred. phenomenon.
4.1.7.5 Certain metals and alloys are subject to a highly
4.1.3 The behavior of certain metals and alloys may be
profoundly influenced by the presence of dissolved oxygen. If localized type of attack called pitting corrosion. This cannot be
evaluated by mass loss alone. The reporting of nonuniform
this is a factor to be considered in a specific test, the solution
should be completely aerated or deaerated in accordance with corrosion is discussed below. It should be appreciated that
pitting is a statistical phenomenon and that the incidence of
8.7.
pitting may be directly related to the area of metal exposed. For
4.1.4 In some cases, the rate of corrosion may be governed
example, a small coupon is not as prone to exhibit pitting as a
by other minor constituents in the solution, in which case they
large one and it is possible to miss the phenomenon altogether
will have to be continually or intermittently replenished by
in the corrosion testing of certain alloys, such as the AISI Type
changing the solution in the test.
300 series stainless steels in chloride contaminated environ-
4.1.5 Corrosion products may have undesirable effects on a
ments.
chemical product. The amount of possible contamination can
4.1.7.6 All metals and alloys are subject to stress-corrosion
be estimated from the loss in mass of the specimen, with proper
cracking under some circumstances. This cracking occurs
application of the expected relationships among (1) the area of
under conditions of applied or residual tensile stress, and it
corroding surface, (2) the mass of the chemical product
may or may not be visible to the unaided eye or upon casual
handled, and (3) the duration of contact of a unit of mass of the
inspection. A metallographic examination may confirm the
chemical product with the corroding surface.
presence of stress-corrosion cracking. It is imperative to note
4.1.6 Corrosion products from the coupon may influence the
that this usually occurs with no significant loss in mass of the
corrosion rate of the metal itself or of different metals exposed
test coupon, although certain refractory metals are an exception
at the same time. For example, the accumulation of cupric ions
to these observations. Generally, if cracking is observed on the
in the testing of copper alloys in intermediate strengths of
coupon, it can be taken as positive indication of susceptibility,
sulfuric acid will accelerate the corrosion of copper alloys, as
whereas failure to effect this phenomenon simply means that it
compared to the rates that would be obtained if the corrosion
did not occur under the duration and specific conditions of the
products were continually removed. Cupric ions may also
test. Separate and special techniques are employed for the
exhibit a passivating effect upon stainless steel coupons ex-
specific evaluation of the susceptibility of metals and alloys to
posed at the same time. In practice, only alloys of the same
stress corrosion cracking (see Ref. (3)).
general type should be exposed in the testing apparatus.
4.1.7 Coupon corrosion testing is predominantly designed
5. Apparatus
to investigate general corrosion. There are a number of other
special types of phenomena of which one must be aware in the
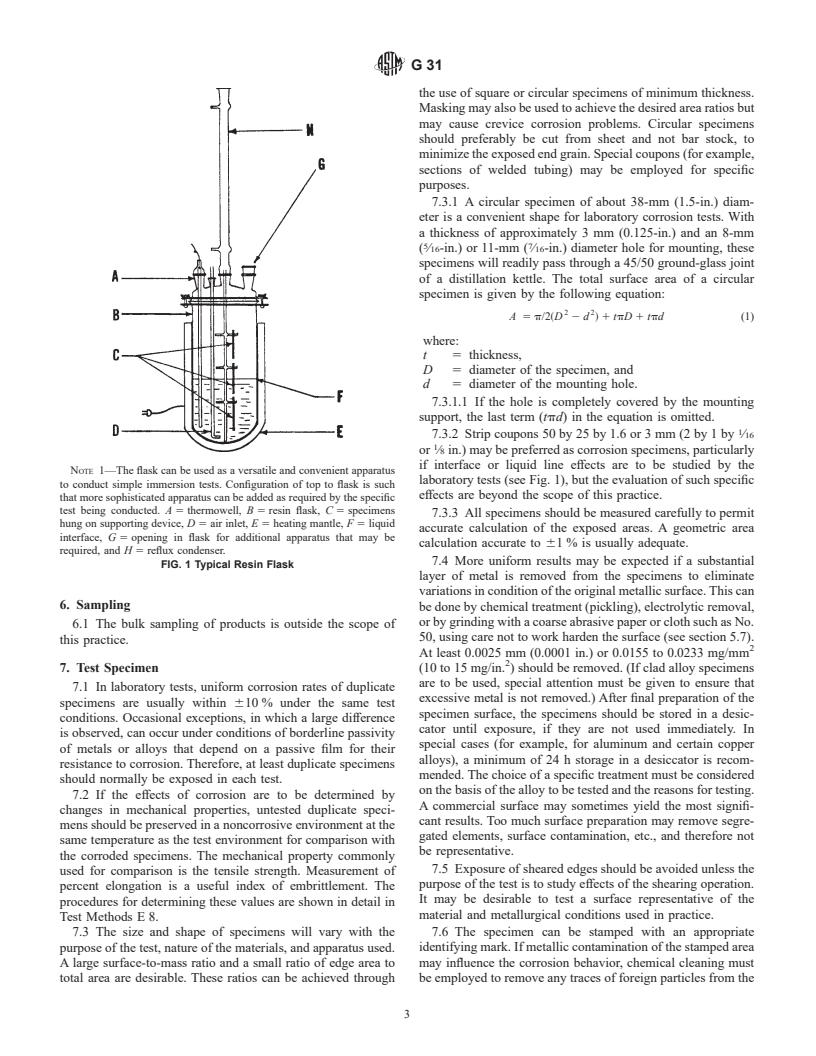
5.1 A versatile and convenient apparatus should be used,
design and interpretation of corrosion tests. consisting of a kettle or flask of suitable size (usually 500 to
4.1.7.1 Galvanic corrosion may be investigated by special 5000 mL), a reflux condenser with atmospheric seal, a sparger
for controlling atmosphere or aeration, a thermowell and
devices which couple one coupon to another in electrical
contact. The behavior of the specimens in this galvanic couple temperature-regulating device, a heating device (mantle, hot
plate, or bath), and a specimen support system. If agitation is
are compared with that of insulated specimens exposed on the
same holder and the galvanic effects noted. It should be required, the apparatus can be modified to accept a suitable
stirring mechanism, such as a magnetic stirrer. A typical resin
observed, however, that galvanic corrosion can be greatly
affected by the area ratios of the respective metals, the distance flask setup for this type test is shown in Fig. 1.
between the metals and the resistivity of the electrolyte. The 5.2 The suggested components can be modified, simplified,
coupling of corrosion coupons then yields only qualitative or made more sophisticated to fit the needs of a particular
results, as a particular coupon reflects only the relationship investigation. The suggested apparatus is basic and the appa-
between these two metals at the particular area ratio involved. ratus is limited only by the judgment and ingenuity of the
investigator.
4.1.7.2 Crevice corrosion or concentration cell corrosion
may occur where the metal surface is partially blocked from 5.2.1 A glass reaction kettle can be used where the configu-
the corroding liquid as under a spacer or supporting hook. It is ration and size of the specimen will permit entry through the
necessary to evaluate this localized corrosion separately from narrow kettle neck (for example, 45/50 ground-glass joint). For
solutions corrosive to glass, suitable metallic or plastic kettles
the overall mass loss.
may be employed.
4.1.7.3 Selective corrosion at the grain boundaries (for
5.2.2 In some cases a wide-mouth jar with a suitable closure
example, intergranular corrosion of sensitized austenitic stain-
less steels) will not be readily observable in mass loss is sufficient when simple immersion tests at ambient tempera-
tures are to be investigated.
measurements unless the attack is severe enough to cause grain
dropping, and often requires microscopic examination of the 5.2.3 Open-beaker tests should not be used because of
coupons after exposure. evaporation and contamination.
4.1.7.4 Dealloying or “parting” corrosion is a condition in 5.2.4 In more complex tests, provisions might be needed for
which one constituent is selectively removed from an alloy, as continuous flow or replenishment of the corrosive liquid, while
in the dezincification of brass or the graphitization of cast iron. simultaneously maintaining a controlled atmosphere.
G31
the use of square or circular specimens of minimum thickness.
Masking may also be used to achieve the desired area ratios but
may cause crevice corrosion problems. Circular specimens
should preferably be cut from sheet and not bar stock, to
minimize the exposed end grain. Special coupons (for example,
sections of welded tubing) may be employed for specific
purposes.
7.3.1 A circular specimen of about 38-mm (1.5-in.) diam-
eter is a convenient shape for laboratory corrosion tests. With
a thickness of approximately 3 mm (0.125-in.) and an 8-mm
5 7
( ⁄16-in.) or 11-mm ( ⁄16-in.) diameter hole for mounting, these
specimens will readily pass through a 45/50 ground-glass joint
of a distillation kettle. The total surface area of a circular
specimen is given by the following equation:
2 2
A 5p/2 D 2 d ! 1 tpD 1 tpd (1)
~
where:
t 5 thickness,
D 5 diameter of the specimen, and
d 5 diameter of the mounting hole.
7.3.1.1 If the hole is completely covered by the mounting
support, the last term (tpd) in the equation is omitted.
7.3.2 Strip coupons 50 by 25 by 1.6 or 3 mm (2 by 1 by ⁄16
or ⁄8 in.) may be preferred as corrosion specimens, particularly
if interface or liquid line effects are to be studied by the
NOTE 1—The flask can be used as a versatile and convenient apparatus
laboratory tests (see Fig. 1), but the evaluation of such specific
to conduct simple immersion tests. Configuration of top to flask is such
effects are beyond the scope of this practice.
that more sophisticated apparatus can be added as required by the specific
test being conducted. A 5 thermowell, B 5 resin flask, C 5 specimens
7.3.3 All specimens should be measured carefully to permit
hung on supporting device, D 5 air inlet, E 5 heating mantle, F 5 liquid
accurate calculation of the exposed areas. A geometric area
interface, G 5 opening in flask for additional apparatus that may be
calculation accurate to 61 % is usually adequate.
required, and H 5 reflux condenser.
7.4 More uniform results may be expected if a substantial
FIG. 1 Typical Resin Flask
layer of metal is removed from the specimens to eliminate
variations in condition of the original metallic surface. This can
6. Sampling
be done by chemical treatment (pickling), electrolytic removal,
or by grinding with a coarse abrasive paper or cloth such as No.
6.1 The bulk sampling of products is outside the scope of
50, using care not to work harden the surface (see section 5.7).
this practice.
At least 0.0025 mm (0.0001 in.) or 0.0155 to 0.0233 mg/mm
(10 to 15 mg/in. ) should be removed. (If clad alloy specimens
7. Test Specimen
are to be used, special attention must be given to ensure that
7.1 In laboratory tests, uniform corrosion rates of duplicate
excessive metal is not removed.) After final preparation of the
specimens are usually within 610 % under the same test
specimen surface, the specimens should be stored in a desic-
conditions. Occasional exceptions, in which a large difference
cator until exposure, if they are not used immediately. In
is observed, can occur under conditions of borderline passivity
special cases (for example, for aluminum and certain copper
of metals or alloys that depend on a passive film for their
alloys
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.