ASTM D1072-90(1999)
(Test Method)Standard Test Method for Total Sulfur in Fuel Gases
Standard Test Method for Total Sulfur in Fuel Gases
SCOPE
1.1 This test method covers the determination of total sulfur in combustible fuel gases, when present in concentrations between 1 and 30 grains of sulfur per 100 cubic feet (25 and 700 mg/m ). It is applicable to natural gases, manufactured gases, and mixed gases, such as are distributed by gas utility companies.
1.2 The values stated in inch-pound units are to be regarded as standard.
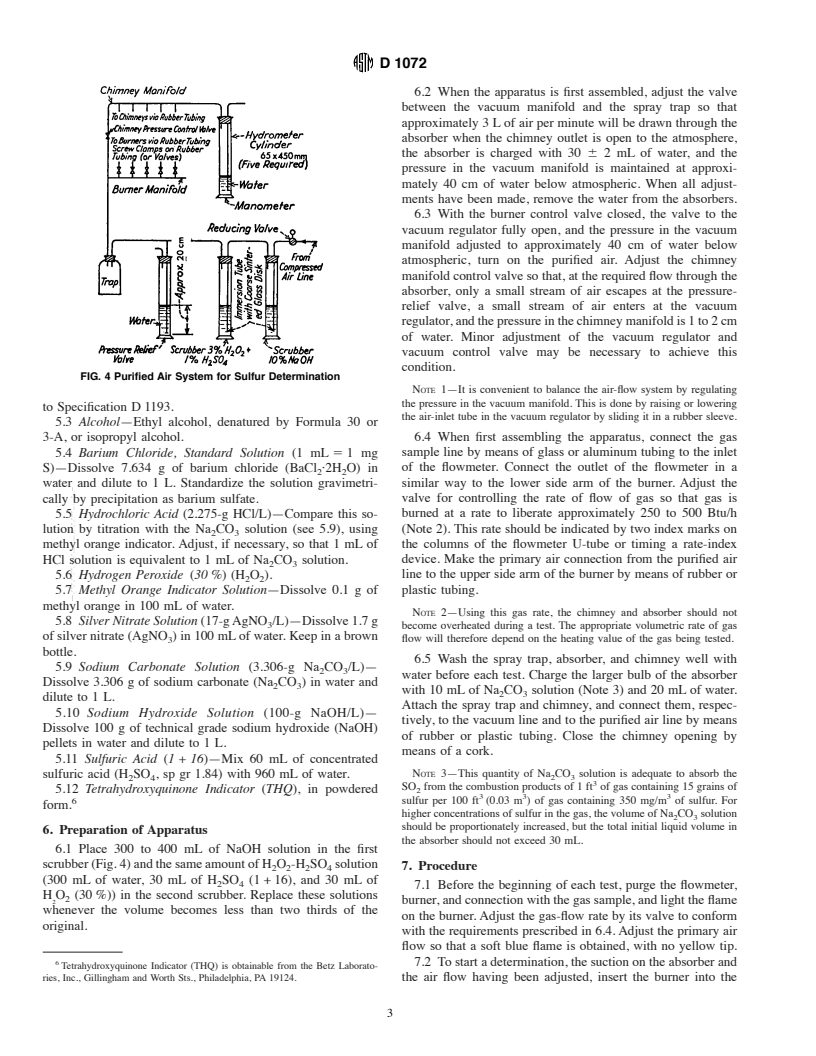
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn
Contact ASTM International (www.astm.org) for the lastest information
Designation:D1072–90(Reapproved 1999)
Standard Test Method for
Total Sulfur in Fuel Gases
This standard is issued under the fixed designation D1072; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope
1.1 Thistestmethodcoversthedeterminationoftotalsulfur
in combustible fuel gases, when present in concentrations
between 1 and 30 grains of sulfur per 100 ft (25 and 700
mg/m ). It is applicable to natural gases, manufactured gases,
and mixed gases, such as are distributed by gas utility
companies.
1.2 Thevaluesstatedininch-poundunitsaretoberegarded
as standard.
1.3 This standard does not purport to address all of the
safety concerns, if any, associated with its use. It is the
responsibility of the user of this standard to establish appro-
priate safety and health practices and determine the applica-
bility of regulatory limitations prior to use.
NOTE 1—All dimensions in millimetres.
2. Referenced Documents
FIG. 1 Gas Burner for Sulfur Determination
2.1 ASTM Standards:
D1193 Specification for Reagent Water
termining and indicating the rate of flow of gas to the burner.
D3031 Test Method for Total Sulfur in Natural Gas by
The capillary selected should be of such size that at the
Hydrogenation
required rate of flow the differential pressure is at least 20 cm
D4468 Test Method for Total Sulfur in Gaseous Fuels by
of water. A scale divided into millimetres will then provide a
Hydrogenolysis and Rateometric Colorimetry
reading precision of 60.5%. Other metering devices, such as
a rotameter or a dry displacement meter, will be suitable if the
3. Summary of Test Method
precision of reading the scale is equivalent.Aflow controlling
3.1 Ameteredsampleofgasisburnedinaclosedsystemin
valve is attached to the inlet connection of the flowmeter.
anatmosphereofsulfur-freeair.Theoxidesofsulfurproduced
4.4 Vacuum System—A vacuum manifold equipped with a
are absorbed in sodium carbonate solution, wherein they are
vacuum regulating device, valves, and so forth. A convenient
oxidized to sulfate. The sulfate in the absorbent solution is
arrangementformultipletestsisshowninFig.3,butanyother
subsequently determined by titration with standard barium
similar system may be used. The system shall be connected to
chloride solution, using tetrahydroxyquinone as an indicator.
a vacuum pump of sufficient capacity to permit a steady gas
flow of 3 L of air per minute through each absorber and to
4. Apparatus
maintain a constant manifold pressure of approximately 40 cm
4.1 Burner (Fig. 1), as specified in the AppendixAppendix
of water below atmospheric.
X1.
4.5 Air-PurifyingSystem—Adevicetosupplypurifiedairto
4.2 Chimneys, Absorbers and Spray Traps, (Fig. 2), as
the burner manifold at a nearly constant pressure of approxi-
specified in the Appendix X1.
mately 20 cm of water and to the chimney manifold at a
4.3 Flowmeter—Acalibratedcapillaryflowmeterforprede-
pressure of 1 to 2 cm of water. A convenient arrangement for
multiple tests is illustrated in Fig. 4, but any other similar
system may be used.The tubing that connects the chimneys to
ThistestmethodisunderthejurisdictionofASTMCommitteeD-3onGaseous
the manifold shall be of an internal diameter not smaller than
Fuels and is the direct responsibility of D03.05 on Determination of Special
0.63 cm to prevent unnecessary restriction of air flow.
Constituents of Gaseous Fuels.
Current edition approved March 30, 1990. Published May 1990. Originally
4.6 Manometer—Awater manometer for indicating the gas
published as D1072–54T. Last previous edition D1072–80.
pressure at the point of volume measurement. It is connected
Annual Book of ASTM Standards, Vol 11.01.
3 betweentheflowmeterandtheburner,withonelegopentothe
Discontinued—See 1991 Annual Book of ASTM Standards, Vol 05.05.
Annual Book of ASTM Standards, Vol 05.06. atmosphere.
Copyright © ASTM, 100 Barr Harbor Drive, West Conshohocken, PA 19428-2959, United States.
D1072
NOTE 1—In the case of those dimensions for which no specific tolerances are designated above, the permissible variation is 610% to the nearest 1
mm, provided, however, that in no case shall the deviation be greater than 5 mm.
FIG. 2 Detailed Drawing of Combustion and Absorption Apparatus for Sulfur Determination
all reagents shall conform to the specifications of the Commit-
tee onAnalytical Reagents of theAmerican Chemical Society,
where such specifications are available. Other grades may be
used, provided it is first ascertained that the reagent is of
sufficiently high purity to permit its use without lessening the
accuracy of the determination.
5.2 PurityofWater—Unlessotherwiseindicated,references
towatershallbeunderstoodtomeanreagentwaterconforming
FIG. 3 Suction System for Sulfur Determination
Reagent Chemicals, American Chemical Society Specifications, American
Chemical Society, Washington, DC. For suggestions on the testing of reagents not
listed by the American Chemical Society, see Analar Standards for Laboratory
5. Reagents and Materials
Chemicals, BDH Ltd., Poole, Dorset, U.K., and the United States Pharmacopeia
5.1 Purity of Reagents—Reagent grade chemicals shall be
and National Formulary, U.S. Pharmacopeial Convention, Inc. (USPC), Rockville,
used in all tests. Unless otherwise indicated, it is intended that MD.
--``,`````,,,,`,`,`````,,```,`-`-`,,`,,`,`,,`---
D1072
6.2 When the apparatus is first assembled, adjust the valve
between the vacuum manifold and the spray trap so that
approximately 3 Lof air per minute will be drawn through the
absorber when the chimney outlet is open to the atmosphere,
the absorber is charged with 30 6 2 mL of water, and the
pressure in the vacuum manifold is maintained at approxi-
mately 40 cm of water below atmospheric. When all adjust-
ments have been made, remove the water from the absorbers.
6.3 With the burner control valve closed, the valve to the
vacuum regulator fully open, and the pressure in the vacuum
manifold adjusted to approximately 40 cm of water below
atmospheric, turn on the purified air. Adjust the chimney
manifoldcontrolvalvesothat,attherequiredflowthroughthe
absorber, only a small stream of air escapes at the pressure-
relief valve, a small stream of air enters at the vacuum
regulator,andthepressureinthechimneymanifoldis1to2cm
of water. Minor adjustment of the vacuum regulator and
vacuum control valve may be necessary to achieve this
condition.
FIG. 4 Purified Air System for Sulfur Determination
NOTE 1—It is convenient to balance the air-flow system by regulating
the pressure in the vacuum manifold. This is done by raising or lowering
to Specification D1193.
the air-inlet tube in the vacuum regulator by sliding it in a rubber sleeve.
5.3 Alcohol—Ethyl alcohol, denatured by Formula 30 or
3-A, or isopropyl alcohol. 6.4 When first assembling the apparatus, connect the gas
5.4 Barium Chloride, Standard Solution (1 mL51 mg sample line by means of glass or aluminum tubing to the inlet
S)—Dissolve 7.634 g of barium chloride (BaCl ·2H O) in of the flowmeter. Connect the outlet of the flowmeter in a
2 2
water and dilute to 1 L. Standardize the solution gravimetri- similar way to the lower side arm of the burner. Adjust the
cally by precipitation as barium sulfate. valve for controlling the rate of flow of gas so that gas is
burned at a rate to liberate approximately 250 to 500 Btu/h
5.5 Hydrochloric Acid (2.275-g HCl/L)—Compare this so-
lution by titration with the Na CO solution (see 5.9), using (Note 2). This rate should be indicated by two index marks on
2 3
the columns of the flowmeter U-tube or timing a rate-index
methyl orange indicator. Adjust, if necessary, so that 1 mL of
HCl solution is equivalent to 1 mL of Na CO solution. device. Make the primary air connection from the purified air
2 3
line to the upper side arm of the burner by means of rubber or
5.6 Hydrogen Peroxide (30%) (H O ).
2 2
5.7 Methyl Orange Indicator Solution—Dissolve 0.1 g of plastic tubing.
methyl orange in 100 mL of water.
NOTE 2—Using this gas rate, the chimney and absorber should not
5.8 SilverNitrateSolution(17-gAgNO /L)—Dissolve1.7g
become overheated during a test. The appropriate volumetric rate of gas
ofsilvernitrate(AgNO )in100mLofwater.Keepinabrown
flow will therefore depend on the heating value of the gas being tested.
bottle.
6.5 Wash the spray trap, absorber, and chimney well with
5.9 Sodium Carbonate Solution (3.306-g Na CO /L)—
2 3
water before each test. Charge the larger bulb of the absorber
Dissolve 3.306 g of sodium carbonate (Na CO ) in water and
2 3
with 10 mLof Na CO solution (Note 3) and 20 mLof water.
2 3
dilute to 1 L.
Attach the spray trap and chimney, and connect them, respec-
5.10 Sodium Hydroxide Solution (100-g NaOH/L)—
tively, to the vacuum line and to the purified air line by means
Dissolve 100 g of technical grade sodium hydroxide (NaOH)
of rubber or plastic tubing. Close the chimney opening by
pellets in water and dilute to 1 L.
means of a cork.
5.11 Sulfuric Acid (1+16)—Mix 60 mL of concentrated
NOTE 3—This quantity of Na CO solution is adequate to absorb the
sulfuric acid (H SO , sp gr 1.84) with 960 mL of water.
2 3
2 4
SO from the combustion products of 1 ft of gas containing 15 grains of
5.12 Tetrahydroxyquinone Indicator (THQ), in powdered 2
3 3 3
6 sulfur per 100 ft (0.03 m ) of gas containing 350 mg/m of sulfur. For
form.
higher concentrations of sulfur in the gas, the volume of Na CO solution
2 3
should be proportionately increased, but the total initial liquid volume in
6. Preparation of Apparatus
the absorbe
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.