ASTM D5972-05(2010)
(Test Method)Standard Test Method for Freezing Point of Aviation Fuels (Automatic Phase Transition Method)
Standard Test Method for Freezing Point of Aviation Fuels (Automatic Phase Transition Method)
SIGNIFICANCE AND USE
The freezing point of an aviation fuel is the lowest temperature at which the fuel remains free of solid hydrocarbon crystals. These crystals can restrict the flow of fuel through the fuel system of the aircraft. The temperature of the fuel in the aircraft tank normally decreases during flight depending on aircraft speed, altitude, and flight duration. The freezing point of the fuel must always be lower than the minimum operational fuel temperature.
Petroleum blending operations require precise measurement of the freezing point.
This test method produces results which have been found to be equivalent to Test Method D2386 and expresses results to the nearest 0.1°C, with improved precision over Test Method D2386. This test method also eliminates most of the operator time and judgment required by Test Method D2386.
When specification requires Test Method D2386, do not substitute this test method or any other test method.
SCOPE
1.1 This test method covers the determination of the temperature below which solid hydrocarbon crystals form in aviation turbine fuels.
1.2 This test method is designed to cover the temperature range of −80 to 20°C; however, 2003 Joint ASTM / IP Interlaboratory Cooperative Test Program mentioned in 12.4 has only demonstrated the test method with fuels having freezing points in the range of −42 to −60°C.
1.3 The values stated in SI units are to be regarded as the standard.
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use. For specific warning statements, see 7.1, 7.3, and 7.5.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: D5972 − 05(Reapproved 2010)
Standard Test Method for
Freezing Point of Aviation Fuels (Automatic Phase
Transition Method)
This standard is issued under the fixed designation D5972; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
This standard has been approved for use by agencies of the U.S. Department of Defense.
1. Scope 3.2.1 automatic phase transition method, n—in this test
method, the procedures of automatically cooling a liquid
1.1 This test method covers the determination of the tem-
aviation fuel specimen until solid hydrocarbon crystals appear,
perature below which solid hydrocarbon crystals form in
followed by controlled warming and recording of the tempera-
aviation turbine fuels.
ture at which the solid hydrocarbon crystals completely redis-
1.2 This test method is designed to cover the temperature
solve into the liquid phase.
range of −80 to 20°C; however, 2003 Joint ASTM / IP
3.2.2 Peltier device, n—a solid-state thermoelectric device
Interlaboratory Cooperative Test Program mentioned in 12.4
constructed with dissimilar semiconductor materials, config-
has only demonstrated the test method with fuels having
ured in such a way that it will transfer heat to and away from
freezing points in the range of −42 to −60°C.
a test specimen dependent on the direction of electric current
1.3 The values stated in SI units are to be regarded as the
applied to the device.
standard.
4. Summary of Test Method
1.4 This standard does not purport to address all of the
4.1 A specimen is cooled at a rate of 15 6 5°C/min by a
safety concerns, if any, associated with its use. It is the
Peltier device while continuously being illuminated by a light
responsibility of the user of this standard to establish appro-
source.Thespecimeniscontinuouslymonitoredbyanarrayof
priate safety and health practices and determine the applica-
optical detectors for the first formation of solid hydrocarbon
bility of regulatory limitations prior to use. For specific
crystals. Once the hydrocarbon crystals are formed, the speci-
warning statements, see 7.1, 7.3, and 7.5.
men is then warmed at a rate of 10 + 0.5°C/min until the last
hydrocarbon crystals return to the liquid phase. The detectors
2. Referenced Documents
are sufficient in number to ensure that any solid hydrocarbon
2.1 ASTM Standards:
crystals are detected. The specimen temperature at which the
D2386Test Method for Freezing Point of Aviation Fuels
last hydrocarbon crystals return to the liquid phase is recorded
as the freezing point.
3. Terminology
5. Significance and Use
3.1 Definitions:
3.1.1 freezing point, n—in aviation fuels, the fuel tempera- 5.1 The freezing point of an aviation fuel is the lowest
ture at which solid hydrocarbon crystals, formed on cooling, temperature at which the fuel remains free of solid hydrocar-
disappear when the temperature of the fuel is allowed to rise boncrystals.Thesecrystalscanrestricttheflowoffuelthrough
the fuel system of the aircraft. The temperature of the fuel in
under specified conditions of test.
theaircrafttanknormallydecreasesduringflightdependingon
3.2 Definitions of Terms Specific to This Standard:
aircraft speed, altitude, and flight duration. The freezing point
ofthefuelmustalwaysbelowerthantheminimumoperational
fuel temperature.
This test method is under the jurisdiction of ASTM Committee D02 on
Petroleum Products, Liquid Fuels, and Lubricants and is the direct responsibility of 5.2 Petroleum blending operations require precise measure-
Subcommittee D02.07 on Flow Properties.
ment of the freezing point.
Current edition approved May 1, 2010. Published May 2010. Originally
ε1
5.3 This test method produces results which have been
approved in 1996. Last previous edition approved in 2005 as D5972–05 . DOI:
10.1520/D5972-05R10.
found to be equivalent to Test Method D2386 and expresses
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
results to the nearest 0.1°C, with improved precision overTest
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Method D2386. This test method also eliminates most of the
Standards volume information, refer to the standard’s Document Summary page on
the ASTM website. operator time and judgment required by Test Method D2386.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
D5972 − 05 (2010)
5.4 When specification requiresTest Method D2386,donot 7.5 Cotton Swabs—Plastic- or paper-shaft cotton swabs to
substitute this test method or any other test method. clean the specimen cup. (Warning—The use of swabs with
wooden shafts may damage the mirrored surface of the
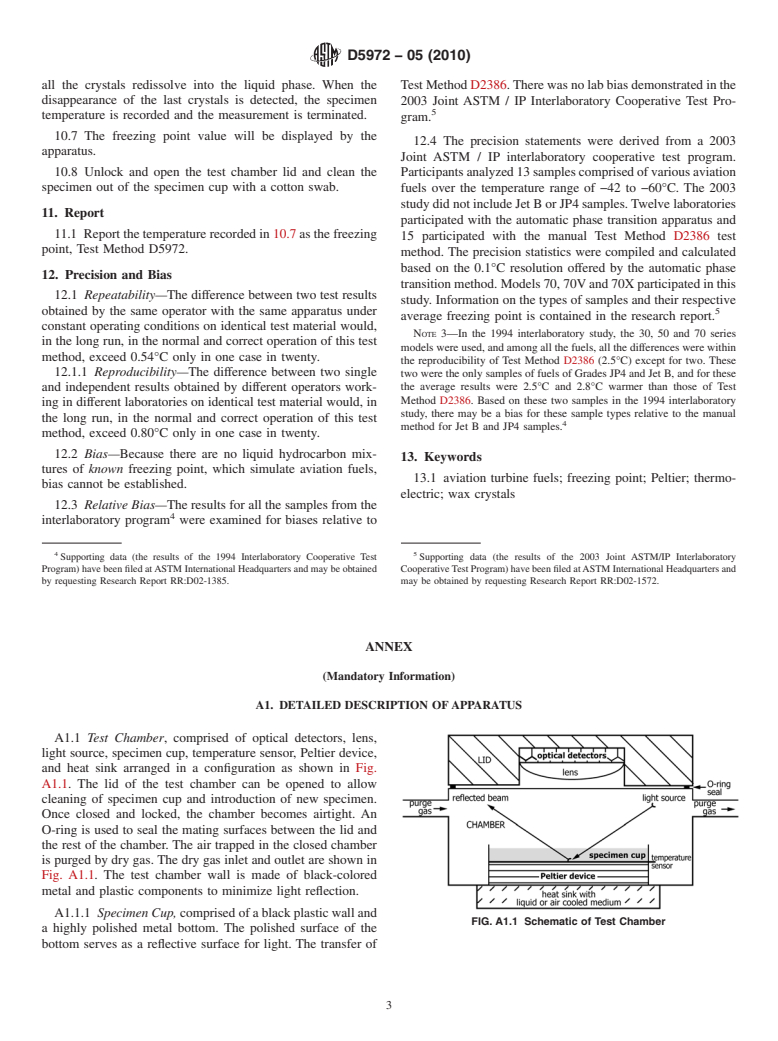
6. Apparatus specimen cup.)
6.1 Automatic Apparatus —This apparatus consists of a
8. Preparation of Apparatus
microprocessor-controlled test chamber that is capable of
8.1 Install the analyzer for operation in accordance with the
cooling and heating the test specimen, optically observing the
manufacturer’s instructions.
appearance and disappearance of solid hydrocarbon crystals,
and recording the temperature of the specimen. A detailed
8.2 Turn on the liquid cooling medium and ensure its
description of the apparatus is provided in Annex A1.
temperature is appropriate for the specimen being tested in
accordance with the manufacturer’s instructions (see Note 1).
6.2 The apparatus shall be equipped with a specimen cup,
optical detector array, light source, digital display, Peltier
8.3 Turn on the purge gas and ensure that it is regulated to
device, and a specimen temperature measuring device.
theappropriatepressureinaccordancewiththemanufacturer’s
instructions.
6.3 The temperature measuring device in the specimen cup
shall be capable of measuring the temperature of the test
8.4 Turn on the main power switch of the analyzer.
specimen from −80 to +20°C at a resolution of 0.1°C and
NOTE 2—Some apparatus are capable of generating a source of dry
accuracy of 0.1°C.
purge gas, thus eliminating the need for an external supply of a
compressed gas.
6.4 The apparatus shall be equipped with fittings to permit
thecirculationofaliquidmediumtoremoveheatgeneratedby
9. Calibration and Standardization
the Peltier device and other electronic components of the
apparatus.
9.1 Ensure that all of the manufacturer’s instructions for
calibrating, checking, and operating the apparatus are fol-
6.5 The apparatus shall be equipped with fittings to permit
lowed.
the circulation of purge gas to purge the test chamber contain-
ing the specimen cup of any atmospheric moisture.
9.2 To verify the performance of the apparatus, an aviation
turbinefuelsampleforwhichextensivedatahasbeenobtained
7. Reagents and Materials
by freeze point, Test Method D2386, may be used. Samples
such as those used in the ASTM interlaboratory cross-check
7.1 n-Octane—Reagent grade is suitable. (Warning—
program would meet this criterion. Such verification materials
Flammable. Harmful if inhaled. Keep away from heat, sparks,
can also be prepared from intracompany cross-checks.
and open flame.)
Alternatively, high-purity n-octane or n-nonane with known
7.2 Cooling Medium—Liquid heat exchange medium to
freezing points can be used to verify the calibration of the
remove the heat generated by the Peltier device and other
temperature-measuring device in the apparatus.
electronic components from the apparatus.
10. Procedure
NOTE 1—Some apparatus are designed to use tap water as a cooling
medium to bring the specimen temperature to −60°C. To achieve cooling
10.1 Open the test chamber lid and clean the specimen cup
of the specimen to −80°C, provide circulation of the cooling medium at
inside the test chamber with a cotton swab.
−30°Corlowertotheapparatus.Sincewaterfreezesat0°C,acommercial
or technical grade isopropanol is suitable as the cooling medium. Refer to
10.2 Rinsethespecimencupbypipetting0.15 60.01mLof
the manufacturer’s operating instructions on the relationship between the
specimen into the cup. Clean the specimen out of the cup by
cooling medium temperature and the minimum specimen temperature.
using a cotton swab. The cup should be cleaned to the point
7.3 Purge Gas—A gas such as air, nitrogen, helium, or
where no visible droplets of specimen remain in the cup.
argon with a dew point below the lowest temperature attained
10.3 Rinse the cup a second time by repeating 10.2.
by the specimen under the conditions of the test. (Warning—
Compressed gas under high pressure.) (Warning—Inert gas 10.4 Carefully measure 0.15 6 0.01 mL of specimen into
can be an asphyxiant when inhaled.) the specimen cup.
7.4 Pipette, capable of dispensing 0.15 6 0.01 mL of 10.5 Close and lock the test chamber lid.
sample.
10.6 Start the operation of the apparatus according to the
manufacturer’s instructions. From this point up to and includ-
ing the termination of the measurement, the apparatus auto-
The sole source of supply of the Phase Technology Freezing Point Analyzer
matically controls all operations. Purge gas and liquid cooling
Model Series 70, 70V, and 70X known to the committee at this time is Phase
medium will begin to flow through the apparatus. The Peltier
Technology, No. 135-11960 Hammersmith Way, Richmond, B.C. Canada, V7A
device cools the specimen at a rate of 15 6 5°C/min. The
5C9. All the model series previously mentioned have identical test chambers and
optical detectors continuously monitor the specimen for the
electronics. The distinction between different model series is the low temperature
limit. Refer to manufacturer’s product information on the low-temperature
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.