ASTM E882-87(2003)
(Guide)Standard Guide for Accountability and Quality Control in the Chemical Analysis Laboratory
Standard Guide for Accountability and Quality Control in the Chemical Analysis Laboratory
SIGNIFICANCE AND USE
An accountability and quality control system is established by laboratory management to improve the quality of its results. It provides documented records which serve to assure users of the laboratory’services that a specified level of precision is achieved in the routine performance of its measurements and that the data reported were obtained from the samples submitted. The system also provides for: early warning to analysts when methods or equipment begin to develop a bias or show deterioration of precision; the protection and retrievability of data (results); traceability and control of samples as they are processed through the laboratory; good communication of sample information between submitters, analysts, and supervision; and information on sample processing history. This guide describes such a system. Other accountability and quality control programs can be developed. Such programs can be equivalent to the program in this guide if they provide all of the benefits mentioned above.
SCOPE
1.1 This guide covers the essential aspects of an accountability and quality control program for a chemical analysis laboratory. The reasons for establishing and operating such a program are discussed.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation:E882–87 (Reapproved 2003)
Standard Guide for
Accountability and Quality Control in the Chemical
Analysis Laboratory
This standard is issued under the fixed designation E882; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope programs can be equivalent to the program in this guide if they
provide all of the benefits mentioned above.
1.1 This guide covers the essential aspects of an account-
ability and quality control program for a chemical analysis
4. Accountability
laboratory. The reasons for establishing and operating such a
4.1 Accountability means assurance that the results reported
program are discussed.
refer directly to the samples submitted.
2. Referenced Documents 4.2 Prior to submitting samples to the laboratory, the pro-
spective user should consult with laboratory personnel con-
2.1 ASTM Standards:
cerning his needs and the capability of the laboratory to satisfy
MNL 7 Manual on Presentation of Data and Control Chart
them. It is the responsibility of the originator of the samples to
Analysis
select and identify proper samples for submission to the
2.2 ANSI Document:
laboratory, to decide what information is required (especially,
ANSI/ASQC A1 Definitions, Symbols, Formulas, and
to define the use to be made of the information), and, after
Tables for Control Charts
consulting with laboratory personnel, to submit the samples in
3. Significance and Use suitable containers, properly labeled, and accompanied by
written instructions identifying the samples, their nature, and
3.1 An accountability and quality control system is estab-
the information sought through chemical analysis. This should
lished by laboratory management to improve the quality of its
be done formally, using a well-defined document for informa-
results. It provides documented records which serve to assure
tion transfer to initiate work in the laboratory.
users of the laboratory’s services that a specified level of
4.3 Laboratory management establishes a written account-
precision is achieved in the routine performance of its mea-
ability system to be used throughout the laboratory at all times.
surements and that the data reported were obtained from the
This implies traceability and documentation of all reported
samples submitted. The system also provides for: early warn-
results through the laboratory back to the submitted sample.
ing to analysts when methods or equipment begin to develop a
This system should have the following general characteristics:
bias or show deterioration of precision; the protection and
4.3.1 Each nonroutine job submitted by a user of the
retrievability of data (results); traceability and control of
laboratory’s services is assigned an internal laboratory identi-
samples as they are processed through the laboratory; good
fication number (ID), which is used to correlate all samples,
communication of sample information between submitters,
work, time and cost accounting, consultation, and reports and
analysts, and supervision; and information on sample process-
other paperwork associated with that job. The final report that
ing history.This guide describes such a system. Other account-
is returned to the originator will always bear the number (ID)
ability and quality control programs can be developed. Such
for future reference. Moreover, it is convenient for laboratory
data to be filed according to sequential ID numbers. For
example, “86/0428” might identify the associated work as the
This guide is under the jurisdiction of ASTM Committee E01 on Analytical
428th request submitted in the year 1986. The Data Record
Chemistry for Metals, Ores, and Related Materials and is the direct responsibility of
shouldprovidealldatageneratedduringtheanalyses,namesof
Subcommittee E01.22 on Laboratory Quality.
persons performing the analyses, dates the analyses were
Current edition approved Oct. 1, 2003. Published November 2003. Originally
approved in 1982. Last previous edition approved in 1998 as E882 – 98. DOI:
performed, and any unusual occurrences that happened during
10.1520/E0882-87R03.
the analyses. Accountability for production control samples is
ASTM Manual Series, ASTM, 6th Edition, 1990.
normally maintained separately from the nonroutine records
Available from American National Standards Institute (ANSI), 25 W. 43rd St.,
4th Floor, New York, NY 10036. because results from production control samples are usually
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
E882–87 (2003)
reported on routine report forms, the samples being identified analytical effort if the method is not initially in control.
with the day, shift, run, or lot from which they were taken. However, when a prolonged series of measurements is made, it
4.3.2 Each sample, specimen, sample site, or other unique is also necessary to verify that the method remains in control
pieceofmaterialorcontaineridentifiedasaseparatesampleby throughout the run.
the originator should be assigned a sequential item number
5.2.4 Provide specific action limits and describe exactly
(NN) for internal laboratory use. As soon as the samples are
what must be done when these limits are exceeded.
accepted by the laboratory, laboratory personnel will mark
5.2.5 For each method (for each sample type), choose a
each sample or sample container with its own laboratory
control material that is known to be stable and homogeneous
sample number (ID-NN) in such manner that the label is not
and has measured values within the range of interest. Any
likely to become separated from its sample or rendered
inhomogeneityinthecontrolsamplewilladdtothevarianceof
unreadable during its residence in the laboratory. For example,
the results.Any increase in variability that is not related to the
the fifth sample on the above-mentioned request might be
measurement process will reduce the sensitivity of the quality
identified as “86/0428-05.”
control procedure to detect changes in the measurement
4.3.3 All laboratory work records, intermediate sample
process. Where possible, the control material should be similar
containers, data, and reports for a specific sample will be
to the samples to be analyzed. Obtain as large an amount of
identified by the same laboratory identification and item
control material as can be prepared in a homogeneous state
number to avoid any opportunity for samples or data to be lost
because considerable effort is required to prepare a new
or intermixed within or between jobs.
control.Always prepare a new control material well in advance
4.3.4 The first and last steps in the accountability procedure
of exhausting the old one so that the new chart is ready when
are functions of technical supervision. Before any work is
needed.Insituationswheresatisfactorycontrolmaterialcannot
performed, the compatibility of the work requested with the
be obtained, alternative techniques (such as, retest by a senior
physical condition of the samples and the capabilities of the
analyst) may be substituted for the control chart approach.
laboratory must be verified. When the analysts have completed
5.2.6 Give analysts specific instructions concerning their
their work, the results must be reviewed to be certain that all
response to an out-of-control condition. Supervision may
information requested has been determined and that the work
decide that, if the analyst can correct the problem so that the
has been performed with the required care and precision. In
control sample results again plot within limits, the process may
this latter regard, quality control procedures prove invaluable
continue without immediate contact with the supervisor. In
both to the analysts performing the work and the reviewing
other situations, the supervisor may need to become involved
supervisor. The supervisor also verifies that the results are
witheachout-of-controlincident.Ineithercase,adjustmentsto
calculated in units that are most meaningful to the submitter
the process should be recorded to explain each shift in the
and that the units and basis on which the results are calculated
control measurements.
are clearly stated.
5.2.7 Provide for a periodic in-depth review by supervision
4.3.5 Except for the most routine work, the original ana-
and management of the overall effectiveness of the laboratory
lyst’s data book, a serial listing of laboratory identification
quality control system. Operating experience may indicate that
numbers and descriptions, and a copy of each job report sheet
methods should be added to, or dropped from the program, that
are retained in the laboratory’s records for the periods of time
the frequency of specific control samples should be increased
established by laboratory policy. Intermediate calculations and
or decreased, or that a different strategy might be more
samples are normally discarded after the submitter has had a
appropriate for control of a specific method. The interval for
reasonable opportunity to submit questions concerning the
such reviews should be determined by the uniformity of the
results and request return of his samples. In some cases,
processes that generate the samples. Any anticipated or ob-
customer specifications may dictate the records that must be
served change in the character of the samples being analyzed
retained and the retention times for both analytical records and
should initiate at least a cursory review of the control proce-
laboratory samples.
dures for the methods that apply to those samples.
5. Quality Control
5.3 Laboratory Quality Control Strategies—Control chart
methods are suitable for laboratory quality control programs.
5.1 Quality control of analytical methods provides the
The choice of which control strategy to use depends on
information needed to ensure that procedures, equipment, and
circumstances: the type of instrument or laboratory procedure,
personnel are performing at the levels of precision and accu-
the number of samples and frequency of the analyses, and the
racy required by the intended use of the data.
closeness of control required. The following are appropriate:
5.2 General Characteristics—The following factors have
¯
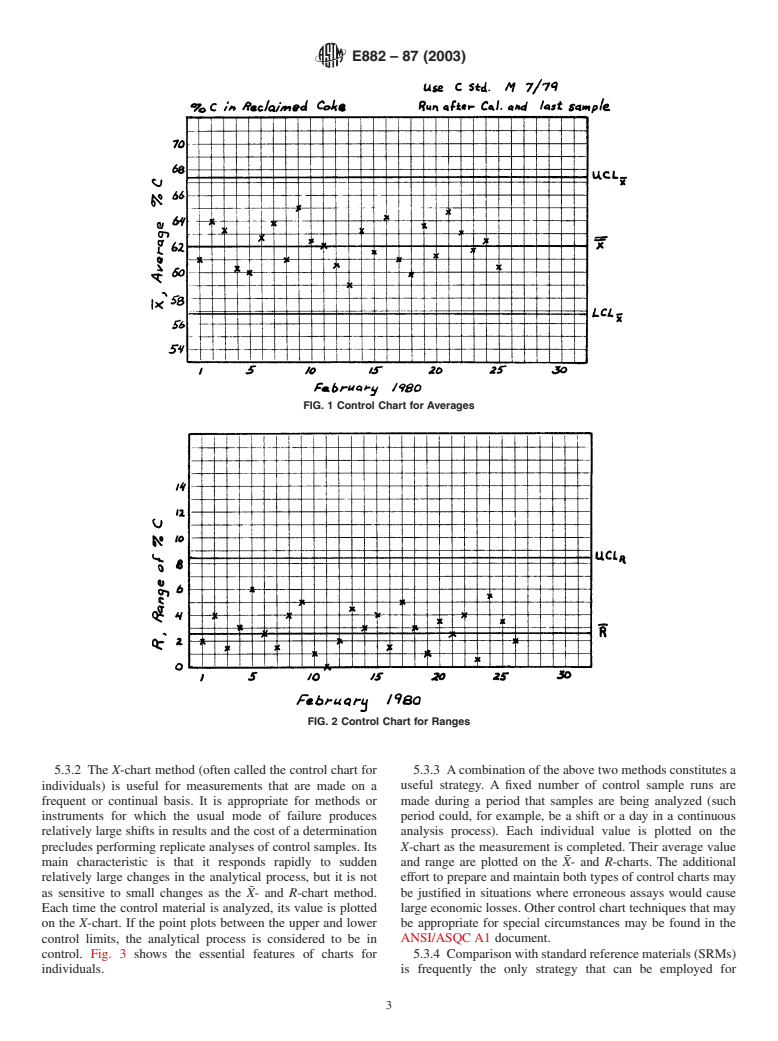
been found helpful in maximizing the effectiveness and mini- 5.3.1 The X- and R-chart method is most frequently used.
mizing the cost of quality control procedures: The control sample is run two or more times during the run,
¯
5.2.1 Involvetheoperatorsoranalystswhoactuallyperform batch, or shift. The average is plotted on the X-chart and the
the work to the greatest possible extent. absolute value of the difference between the high and low
5.2.2 Use the simplest, most direct statistical procedures values, the range, is plotted on the R-chart. If the average falls
that will provide the necessary degree of control. This means between the upper and lower control limits and the range falls
thatgraphicalorsimplifiedarithmeticproceduresarepreferred. belowtheuppercontrollimit,theprocessisconsideredtobein
5.2.3 Perform the quality control measurements as early in control. Fig. 1 shows the essential features of charts for
the measurement process as possible. This prevents waste of averages and ranges.
E882–87 (2003)
FIG. 1 Control Chart for Averages
FIG. 2 Control Chart for Ranges
5.3.2 The X-chart method (often called the control chart for 5.3.3 Acombination of the above two methods constitutes a
individuals) is useful for measurements that are made on a useful strategy. A fixed number of control sample runs are
frequent or continual basis. It is appropriate for methods or made during a period that samples are being analyzed (such
instruments for which the usual mode of failure produces period could, for example, be a shift or a day in a continuous
relatively large shifts in results and the cost of a determination analysis process). Each individual value is plotted on the
precludes performing replicate analyses of control samples. Its X-chart as the measurement is completed. Their average value
¯
main characteristic is that it responds rapidly to sudden and range are plotted on the X- and R-charts. The additional
relatively large changes in the analytical process, but it is not effort to prepare and maintain both types of control charts may
¯
as sensitive to small changes as the X- and R-chart method. be justified in situations where erroneous assays would cause
Each time the control material is analyzed, its value is plotted large economic losses. Other control chart techniques that may
on the X-chart. If the point plots between the upper and lower be appropriate for special circumstances may be found in the
control limits, the analytical process is considered to be in ANSI/ASQC A1 document.
control. Fig. 3 shows the essential features of charts for 5.3.4 Comparisonwithstandardreferencematerials(SRMs)
individuals. is frequently the only strategy that can be employed for
E882–87 (2003)
FIG. 3 Control Chart for Individuals
infrequently used analytical methods or for nonroutine sample 5.5 Control Chart Construction—Calculate the central
types. If an SRM such as one from the National Institute of value and control limits. Prepare the control chart with the
Standards andTechnology (similar to the samples) is run along vertical scale labeled so that the central value is approximately
with the samples, comparison of the measured value against midway on the graph. Select a scale factor to permit the results
the known value of the standard provides a measure of to be plotted accurately and easily. The horizontal axis is
confidence in the sample assays. Lacking an SRM, any labeled by shift, day, run number, or run date, as appropriate.
previously analyzed material may be used. In all cases, it is Draw and label the central line and the lines representing the
important to retain as large a portion of such a material as upper and lower control limits. The central line may be the
possible and to tabulate the results, the method used, the date, grand average of the values obtained during the base period
and the analyst. Materials and data thus obtained may have described below, or it may be a specifed value based upon
important future statistical or control chart use. experience. Title the chart with the method, the control sample
5.4 Definitions: identification, and any special instructions. Show all of the
5.4.1 mean: original data points that were used to calculate the control
limits.
¯
X 5 X 1 X 1
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.