ASTM F2255-05
(Test Method)Standard Test Method for Strength Properties of Tissue Adhesives in Lap-Shear by Tension Loading
Standard Test Method for Strength Properties of Tissue Adhesives in Lap-Shear by Tension Loading
SIGNIFICANCE AND USE
Materials and devices that function at least in part by adhering to living tissues are finding increasing use in surgical procedures either as adjuncts to sutures and staples, or as frank replacements for those devices in a wide variety of medical procedures. While the nature and magnitude of the forces involved varies greatly with indication and with patient specific circumstances, all uses involve to some extent the ability of the material to resist imposed mechanical forces. Therefore, the mechanical properties of the materials, and in particular the adhesive properties, are important parameters in evaluating their fitness for use. In addition, the mechanical properties of a given adhesive composition can provide a useful means of determining product consistency for quality control, or as a means for determining the effects of various surface treatments on the substrate prior to use of the device.
The complexity and variety of individual applications for tissue adhesive devices, even within a single indicated use (surgical procedure) is such that the results of a single-lap-shear test are not suitable for determining allowable design stresses without thorough analysis and understanding of the application and adhesive behaviors.
This test method may be used for comparing adhesives or bonding processes for susceptibility to fatigue and environmental changes, but such comparisons must be made with great caution since different adhesives may respond differently to varying conditions.
SCOPE
1.1 This test method is intended to provide a means for comparison of the adhesive strengths of tissue adhesives intended for use as surgical adhesives or sealants, or both, on soft tissue. With the appropriate choice of substrate, it may also be used for purposes of quality control in the manufacture of tissue adhesive based medical devices.
1.2 The values stated in SI units are to be regarded as the standard.
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: F2255 – 05
Standard Test Method for
Strength Properties of Tissue Adhesives in Lap-Shear by
1
Tension Loading
This standard is issued under the fixed designation F2255; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope for use in closing wounds (surgical or traumatic) or for sealing
against leakage of body fluids.
1.1 This test method is intended to provide a means for
3.2.2 tissue sealant—a surface coating with adequate adhe-
comparison of the adhesive strengths of tissue adhesives
sive strength to prevent leakage of body fluids.
intended for use as surgical adhesives or sealants, or both, on
softtissue.Withtheappropriatechoiceofsubstrate,itmayalso
4. Significance and Use
be used for purposes of quality control in the manufacture of
4.1 Materials and devices that function at least in part by
tissue adhesive based medical devices.
adhering to living tissues are finding increasing use in surgical
1.2 The values stated in SI units are to be regarded as the
procedures either as adjuncts to sutures and staples, or as frank
standard.
replacements for those devices in a wide variety of medical
1.3 This standard does not purport to address all of the
procedures. While the nature and magnitude of the forces
safety concerns, if any, associated with its use. It is the
involvedvariesgreatlywithindicationandwithpatientspecific
responsibility of the user of this standard to establish appro-
circumstances, all uses involve to some extent the ability of the
priate safety and health practices and determine the applica-
material to resist imposed mechanical forces. Therefore, the
bility of regulatory limitations prior to use.
mechanical properties of the materials, and in particular the
2. Referenced Documents adhesive properties, are important parameters in evaluating
2
their fitness for use. In addition, the mechanical properties of a
2.1 ASTM Standards:
given adhesive composition can provide a useful means of
D907 Terminology of Adhesives
determining product consistency for quality control, or as a
E4 Practices for Force Verification of Testing Machines
3
means for determining the effects of various surface treatments
2.2 American Association of Tissue Banks Standards:
on the substrate prior to use of the device.
Standards for Tissue Banking
4.2 The complexity and variety of individual applications
3. Terminology
for tissue adhesive devices, even within a single indicated use
(surgical procedure) is such that the results of a single-lap-
3.1 Definitions—Many terms in this test method are defined
shear test are not suitable for determining allowable design
in Terminology D907.
stresses without thorough analysis and understanding of the
3.2 Definitions:
application and adhesive behaviors.
3.2.1 tissue adhesive—for the purposes of this test method,
4.3 This test method may be used for comparing adhesives
tissue adhesive is defined as a compound or system intended
or bonding processes for susceptibility to fatigue and environ-
mentalchanges,butsuchcomparisonsmustbemadewithgreat
caution since different adhesives may respond differently to
1
This test method is under the jurisdiction ofASTM Committee F04 on Medical
varying conditions.
and Surgical Materials and Devices and is the direct responsibility of Subcommittee
F04.15 on Material Test Methods.
Current edition approved Mar. 1, 2005. Published March 2005. Originally 5. Apparatus
approved in 2003. Last previous edition approved in 2003 as F2255 – 03. DOI:
5.1 Testing Machine, of the constant-rate-of-crosshead-
10.1520/F2255-05.
2
movement type and comprising essentially the following:
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
5.1.1 Fixed Member, a fixed or essentially stationary mem-
Standards volume information, refer to the standard’s Document Summary page on
ber carrying one grip.
the ASTM website.
3 5.1.2 Movable Member, a movable member carrying a
Available from the American Association of Tissue Banks (AATB), 1350
Beverly Rd., Suite 220-A, McLean, VA 22101. second grip.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
1
---------------------- Page: 1 ----------------------
F2255 – 05
5.1.3 Grips, for holding the test specimen between the fixed be brought to the test temperature or other prescribed tempera-
member and the movable member of the testing machine can ture (such as body temperature) prior to application of the
be either the fixed or self-aligning type. adhesive.
5.1.3.
...







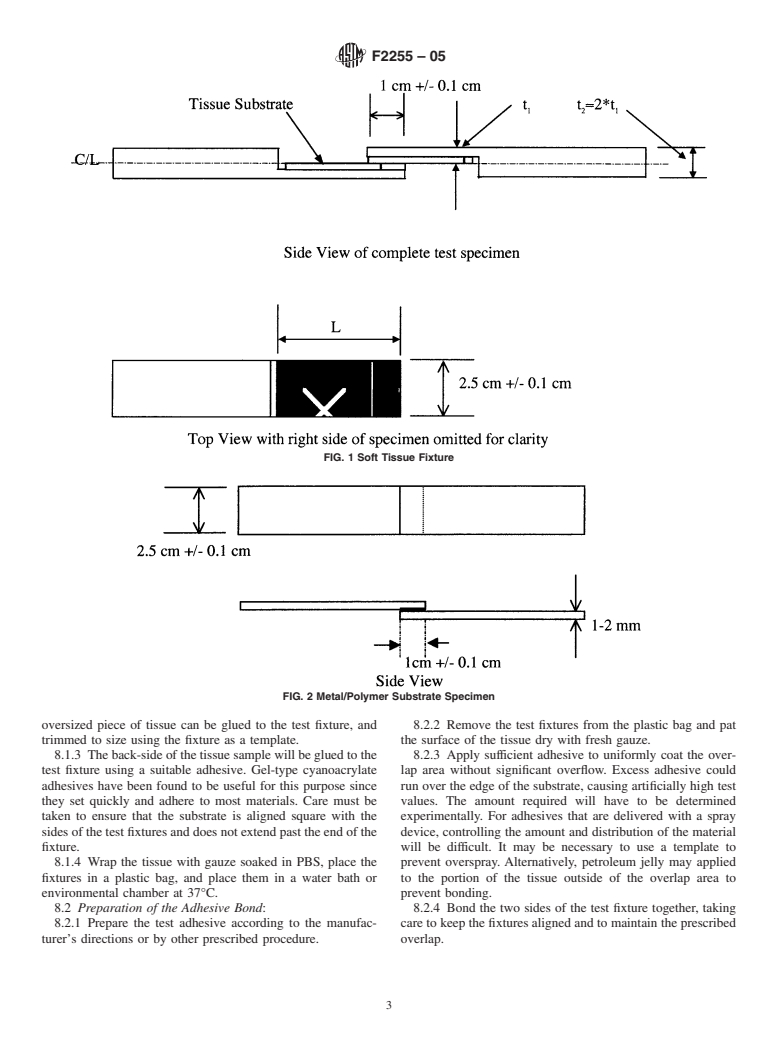
Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.