ASTM D6877-03
(Test Method)Standard Test Method for Monitoring Diesel Particulate Exhaust in the Workplace
Standard Test Method for Monitoring Diesel Particulate Exhaust in the Workplace
SCOPE
1.1 This test method covers determination of organic and elemental carbon in the particulate fraction of diesel engine exhaust, hereafter referred to as diesel particulate matter (DPM). Samples of workplace atmospheres are collected on quartz-fiber filters. The method also is suitable for other types of carbonaceous aerosols, but it is not appropriate for sampling volatile or semi-volatile components. These components require sorbents for efficient collection.
Note 1—Sample collection and handling procedures for environmental samples differ from occupational samples. This standard addresses occupational monitoring of DPM in workplaces where diesel-powered equipment is used.
1.2 The method is based on a thermal-optical technique (1, 2). Speciation of organic and elemental carbon is achieved through temperature and atmosphere control, and an optical feature that corrects for sample charring.
1.3 A portion of a 37-mm, quartz-fiber filter sample is analyzed. Results for the portion are used to calculate the total mass of organic and elemental carbon on the filter. The portion must be representative of the entire filter deposit. If the deposit is uneven, two or more representative portions should be analyzed for an average. Open-faced cassettes give even deposits but are often not practical. Closed-face cassettes give equivalent results if other dusts are absent. Other samplers may be required, depending on the sampling environment (2-5).
1.4 The calculated limit of detection ( LOD) depends on the level of contamination of the media blanks (). A LOD of approximately 0.2 g carbon per cm2 of filter was estimated when analyzing a sucrose standard solution applied to filter portions cleaned immediately before analysis. LODs based on media blanks stored after cleaning are usually higher. LODs based on a set of media blanks from a commercial laboratory were OC = 1.2 g/cm2, EC = 0.4 g/cm2, and TC = 1.3 g/cm 2, where OC, EC, and TC refer to organic, elemental, and total carbon, respectively.
1.5 OC-EC methods are operational, which means the analytical procedure defines the analyte. The test method offers greater selectivity and precision than thermal techniques that do not correct for charring of organic components. The analysis method is simple and relatively quick (about 15 min). The analysis and data reduction are automated, and the instrument is programmable (different methods can be saved as methods for other applications).
1.6 A method (5040) for DPM based on thermal-optical analysis has been published by the National Institute for Occupational Safety and Health (NIOSH). Method updates (3, 4) have been published since its initial (1996) publication in the NIOSH Manual of Analytical Methods (NMAM). Both OC and EC are determined by NMAM 5040. An EC exposure marker was recommended because EC is a more selective measure of exposure. A comprehensive review of the method and rationale for selection of an EC marker are provided in a recent Chapter of NMAM (5).
1.7 The thermal-optical instrument required for the analysis is manufactured by a private laboratory. As with most instrumentation, design improvements continue to be made. Different laboratories may be using different instrument models.
1.8 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use. Specific precautionary statements are given in 7.1.5, 8.3, and 12.12.2.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation:D6877–03
Standard Test Method for
Monitoring Diesel Particulate Exhaust in the Workplace
This standard is issued under the fixed designation D 6877; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope greater selectivity and precision than thermal techniques that
donotcorrectforcharringoforganiccomponents.Theanalysis
1.1 This test method covers determination of organic and
method is simple and relatively quick (about 15 min). The
elemental carbon in the particulate fraction of diesel engine
analysis and data reduction are automated, and the instrument
exhaust, hereafter referred to as diesel particulate matter
is programmable (different methods can be saved as methods
(DPM). Samples of workplace atmospheres are collected on
for other applications).
quartz-fiber filters. The method also is suitable for other types
1.6 A method (5040) for DPM based on thermal-optical
of carbonaceous aerosols, but it is not appropriate for sampling
analysis has been published by the National Institute for
volatile or semi-volatile components. These components re-
Occupational Safety and Health (NIOSH). Method updates (3,
quire sorbents for efficient collection.
4)havebeenpublishedsinceitsinitial(1996)publicationinthe
NOTE 1—Samplecollectionandhandlingproceduresforenvironmental
NIOSH Manual ofAnalytical Methods (NMAM). Both OC and
samples differ from occupational samples. This standard addresses occu-
EC are determined by NMAM 5040. An EC exposure marker
pational monitoring of DPM in workplaces where diesel-powered equip-
was recommended because EC is a more selective measure of
ment is used.
exposure.Acomprehensive review of the method and rationale
1.2 The method is based on a thermal-optical technique (1,
for selection of an EC marker are provided in a recent Chapter
2) . Speciation of organic and elemental carbon is achieved
of NMAM (5).
through temperature and atmosphere control, and an optical
1.7 The thermal-optical instrument required for the analysis
feature that corrects for sample charring. 3
is manufactured by a private laboratory. As with most instru-
1.3 A portion of a 37-mm, quartz-fiber filter sample is
mentation, design improvements continue to be made. Differ-
analyzed. Results for the portion are used to calculate the total
ent laboratories may be using different instrument models.
mass of organic and elemental carbon on the filter.The portion
1.8 This standard does not purport to address all of the
must be representative of the entire filter deposit. If the deposit
safety concerns, if any, associated with its use. It is the
is uneven, two or more representative portions should be
responsibility of the user of this standard to establish appro-
analyzed for an average. Open-faced cassettes give even
priate safety and health practices and determine the applica-
deposits but are often not practical. Closed-face cassettes give
bility of regulatory limitations prior to use. Specific precau-
equivalentresultsifotherdustsareabsent.Othersamplersmay
tionary statements are given in 7.1.5, 8.3, and 12.12.2.
be required, depending on the sampling environment (2-5).
1.4 The calculated limit of detection (LOD) depends on the
2. Referenced Documents
level of contamination of the media blanks (5). A LOD of 4
2.1 ASTM Standards:
approximately 0.2 µg carbon per cm of filter was estimated
D 1356 Terminology Relating to Sampling and Analysis of
when analyzing a sucrose standard solution applied to filter
Atmospheres
portions cleaned immediately before analysis. LODs based on
media blanks stored after cleaning are usually higher. LODs 3. Terminology
based on a set of media blanks from a commercial laboratory
3.1 Definitions:
2 2 2
were OC=1.2µg/cm , EC=0.4µg/cm ,and TC=1.3µg/cm ,
where OC, EC, and TC refer to organic, elemental, and total
carbon, respectively.
The carbon analyzer used in the development and performance evaluation of
1.5 OC-EC methods are operational, which means the th
this test method was manufactured by Sunset Laboratory, 2017 19 Avenue, Forest
analyticalproceduredefinestheanalyte.Thetestmethodoffers Grove, Oregon 97116, which is the sole source of supply of the instrument known
to the committee at this time. If you are aware of alternative suppliers, please
provide this information to ASTM Headquarters. Your comments will receive
This test method is under the jurisdiction of ASTM Committee D22 on carefulconsiderationatameetingoftheresponsibletechnicalcommitteewhichyou
Sampling and Analysis of Atmospheres and is the direct responsibility of Subcom- may attend.
mittee D22.04 on Workplace Atmospheres. For referenced ASTM standards, visit the ASTM website, www.astm.org, or
Current edition approved May 10, 2003. Published June 2003. contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
The boldface numbers in parentheses refer to references at the end of this test Standards volume information, refer to the standard’s Document Summary page on
method. the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
D6877–03
3.2 For definitions of terms used in this practice, refer to control. In addition, the analyzer is equipped with an optical
Terminology D 1356. feature that corrects for the char formed during the analysis of
3.3 limit of detection, LOD—Avalue for which exceedence some materials. Optical correction is made with a pulsed diode
by measured mass indicates the presence of a substance at laser and photodetector that permit continuous monitoring of
given false-positive rate: 3 3 estimated standard deviation of the filter transmittance.
estimated mass. 4.2 The main instrument components are illustrated in Fig.
3.4 Definitions of Terms Specific to This Standard:
1. The instrument output, called a thermogram, is shown in
3.4.1 organic carbon (OC)—Carbon volatilized in helium Fig. 2. For analysis, a known area (normally 1.5 cm)ofthe
while heating a quartz-fiber filter sample to 870°C. Includes
quartz-fiber filter sample is removed with a sharp metal punch.
carbonates, if present, unless quantified separately. Also in- Quartz-fiber filters are required because temperatures in excess
cludes char formed during pyrolysis of some materials.
of 850°C are employed.The portion is inserted into the sample
3.4.2 elemental carbon (EC)—Excluding char, light- oven, and the oven is tightly sealed. The analysis proceeds in
absorbing carbon that is not removed from a filter sample
inert and oxidizing atmospheres. First, OC (and carbonate, if
heated to 870°C in an inert atmosphere. present) is removed in helium as the temperature is stepped to
3.4.3 total carbon (TC)—Sum of organic and elemental a preset maximum (about 870°C in NMAM 5040). Evolved
carbon.
carbon is catalytically oxidized to CO in a bed of granular
3.4.4 thermogram—Digitized output signal of thermal- MnO . The CO is then reduced to CH in a Ni/firebrick
2 2 4
optical instrument. Shows detector and filter transmittance
methanator, and CH is quantified by a FID. Next, the sample
signals at different temperatures in nonoxidizing and oxidizing oven temperature is lowered, an oxygen-helium mix (2 %
atmospheres.
oxygen after dilution of the 10 % oxygen in helium supply) is
3.5 Symbols and Abbreviations: introduced, and the temperature is increased to 900°C (or
3.5.1 DPM—diesel particulate matter
higher) to remove the residual carbon. At the end of each
3.5.2 LOD (µg/cm )—limit of detection: 3 3 s analysis, calibration is made through automatic injection of a
w
3.5.3 s (µg/cm )—estimate of sw
fixed volume of methane.
w
3.5.4 s (µg/cm )—standard deviation in collected mass
4.3 Some samples contain components (for example, ciga-
w
loading determination
rette and wood smokes) that carbonize (convert to carbon) or
3.5.5 OC, EC, TC (µg/cm or µg)—organic, elemental, and
char in helium during the first part of the analysis. Like EC
total carbon
initially present in the sample, char strongly absorbs light,
3.5.6 RSD—relative standard deviation
particularlyinthered/infraredregion.Thecharformedthrough
3.5.7 V (L)—sampled volume
pyrolysis (thermal decomposition) of these components causes
3.5.8 W (µg)—field blank filter’s EC mass reading
the filter transmittance to decrease. Charring can begin at
b
3.5.9 W (µg)—active filter’s EC mass reading
300°C; the process may continue until the maximum tempera-
EC
ture is reached. After OC removal, an oxygen-helium mix is
4. Summary of Test Method
introduced to effect combustion of residual carbon, which
4.1 The thermal-optical analyzer has been described previ- includes char and any EC originally present.As oxygen enters
ously (1-5). Design improvements have been made over time, the oven, light-absorbing carbon is oxidized and a concurrent
but the operation principle remains unchanged. OC-EC quan- increase in filter transmittance occurs. The split (vertical line
tification is accomplished through temperature and atmosphere prior to EC peak in Fig. 2) between OC and EC is assigned
FIG. 1 Schematic of Thermal-Optical Instrument (V=valve) for Determination of Organic and Elemental Carbon in DPM and Other
Carbonaceous Aerosols.
D6877–03
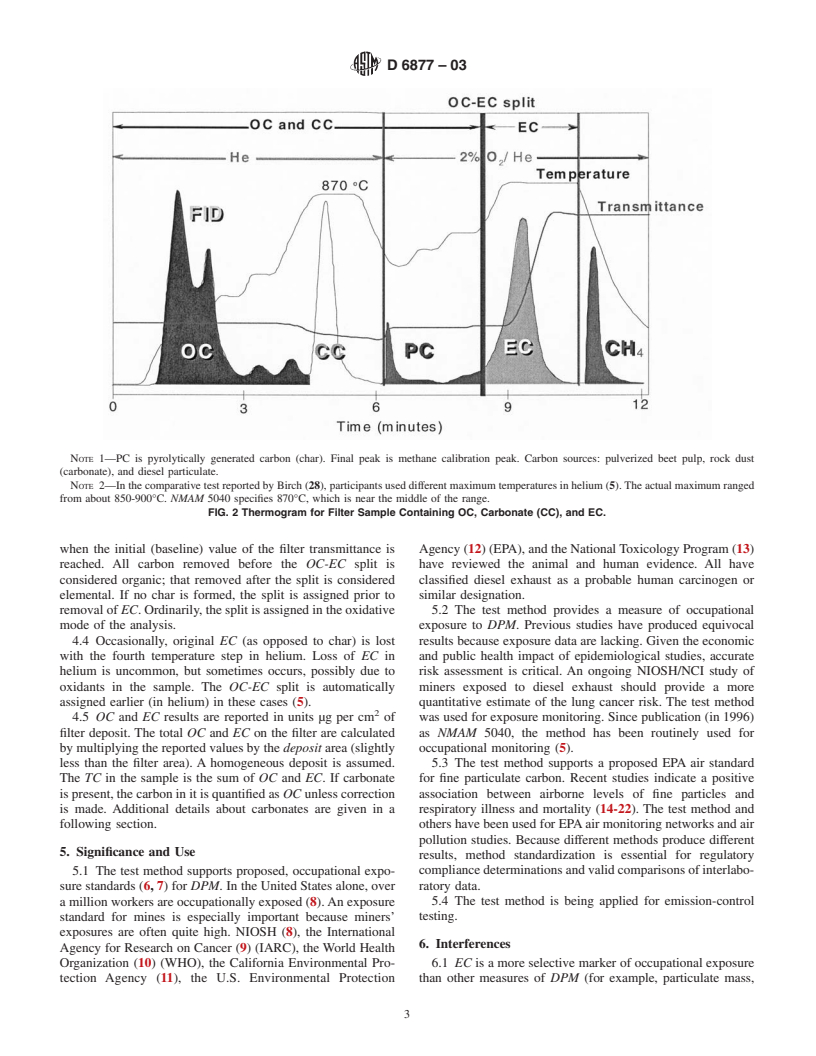
NOTE 1—PC is pyrolytically generated carbon (char). Final peak is methane calibration peak. Carbon sources: pulverized beet pulp, rock dust
(carbonate), and diesel particulate.
NOTE 2—In the comparative test reported by Birch (28), participants used different maximum temperatures in helium (5).The actual maximum ranged
from about 850-900°C. NMAM 5040 specifies 870°C, which is near the middle of the range.
FIG. 2 Thermogram for Filter Sample Containing OC, Carbonate (CC), and EC.
when the initial (baseline) value of the filter transmittance is Agency (12) (EPA), and the NationalToxicology Program (13)
reached. All carbon removed before the OC-EC split is have reviewed the animal and human evidence. All have
considered organic; that removed after the split is considered classified diesel exhaust as a probable human carcinogen or
elemental. If no char is formed, the split is assigned prior to similar designation.
removal of EC. Ordinarily, the split is assigned in the oxidative 5.2 The test method provides a measure of occupational
mode of the analysis. exposure to DPM. Previous studies have produced equivocal
4.4 Occasionally, original EC (as opposed to char) is lost results because exposure data are lacking. Given the economic
with the fourth temperature step in helium. Loss of EC in and public health impact of epidemiological studies, accurate
helium is uncommon, but sometimes occurs, possibly due to risk assessment is critical. An ongoing NIOSH/NCI study of
oxidants in the sample. The OC-EC split is automatically miners exposed to diesel exhaust should provide a more
assigned earlier (in helium) in these cases (5). quantitative estimate of the lung cancer risk. The test method
4.5 OC and EC results are reported in units µg per cm of was used for exposure monitoring. Since publication (in 1996)
filter deposit. The total OC and EC on the filter are calculated as NMAM 5040, the method has been routinely used for
by multiplying the reported values by the deposit area (slightly occupational monitoring (5).
less than the filter area). A homogeneous deposit is assumed. 5.3 The test method supports a proposed EPA air standard
The TC in the sample is the sum of OC and EC. If carbonate for fine particulate carbon. Recent studies indicate a positive
is present, the carbon in it is quantified as OC unless correction association between airborne levels of fine particles and
is made. Additional details about carbonates are given in a respiratory illness and mortality (14-22). The test method and
following section. others have been used for EPAair monitoring networks and air
pollution studies. Because different methods produce different
5. Significance and Use
results, method standardization is essential for regulatory
compliancedeterminationsandvalidcomparisonsofinterlabo-
5.1 The test method supports proposed, occupational expo-
sure standards (6, 7) for DPM. In the United States alone, over ratory data.
5.4 The test method is being applied for emission-control
a million workers are occupationally exposed (8).An exposure
standard for mines is especially important because miners’ testing.
exposures are often quite high. NIOSH (8), the International
6. Interferences
Agency for Research on Cancer (9) (IARC), the World Health
Organization (10) (WHO), the California Environmental Pro- 6.1 EC is a more selective marker of occupational exposure
tection Agency (11), the U.S. Environmental Protection than other measures of DPM (for example, particulate mass,
D6877–03
total carbon). As defined by the test method, EC is the carbon 7.1.6 Valve box/calibration loop—for control of gas flow
determined during the second stage of the analysis (after and automatic injection of methane internal standard.
pyrolysis correction). If the sample contains no pyrolyzable
8. Reagents and Materials
material, all carbon evolved during this stage is considered
elemental. Inorganic dusts, carbonates, and wood and cigarette
8.1 Organic Carbon (OC) Standards—Sucrose stock solu-
smokes ordinarily do not interfere in the EC determination
tion having carbon concentration of 25 mg/mL. Working
(2-5). OC can be contributed by smokes, fumes and other
standards (dilutions of stock) with concentrations of 0.1 to 3
sources.
mg C per mL solution. Ensure carbon loadings of standards
6.2 If high levels of other dusts are present, a size classifier
spiked onto filter punches bracket the range of the samples.
(for example, impactor, or cyclone, or both) should be used. If
8.2 Ultrapure water, Type I, (for preparation of sucrose
the dust is carbonaceous, a size classifier provides a more
standard solution).
selective measure of the diesel-source OC. It also provides a
8.3 Sucrose—reagent grade (99+ %).
better measure of the diesel-source EC if the dust contains EC
8.4 Helium-UHP (99.999%). Scrubber also required for
(for example, carbon black, coal), which is less common. A
removal of trace oxygen.
finely ground sample of the bulk material can be analyzed to
8.5 Hydrogen—purified (99.995%). Cylinder or hydrogen
determinewhetheradustposespotentialinterference.Depend-
generator source. Warning—Hydrogen is a flammable gas.
ing on the dust concentration, size distribution, and target
Users must be familiar
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.