ASTM D5198-17
(Practice)Standard Practice for Nitric Acid Digestion of Solid Waste
Standard Practice for Nitric Acid Digestion of Solid Waste
SIGNIFICANCE AND USE
5.1 A knowledge of the inorganic composition of a waste is often required for the selection of appropriate waste disposal practices. Solid waste may exist in a variety of forms and contain a range of organic and inorganic constituents. This practice describes a digestion procedure which dissolves many of the toxic inorganic constituents and produces a solution suitable for determination of total recoverable contents by such techniques as atomic absorption spectroscopy, atomic emission spectroscopy, and so forth. The relatively large sample size aids representative sampling of heterogenous wastes. The relatively small dilution factor allows lower detection limits than most other sample digestion methods. Volatile metals, such as lead and mercury, are not lost during this digestion procedure, however organo-lead and organo-mercury may not be completely digested. Hydride-forming elements, such as arsenic and selenium, may be partially lost. Samples with total metal contents greater than 5 % may give low results. The analyst is responsible for determining whether this practice is applicable to the solid waste being tested.
SCOPE
1.1 This practice describes the partial digestion of solid waste using nitric acid for the subsequent determination of the total recoverable content of inorganic constituents.
1.2 This practice is to be used when the concentrations of total recoverable elements are to be determined from a waste sample. Total recoverable elements are often not equivalent to total elemental content, because of the solubility of the speciated forms of the element in the sample matrix. Recovery from refractory sample matrices, such as soils, is usually significantly less than total concentrations of the elements present.
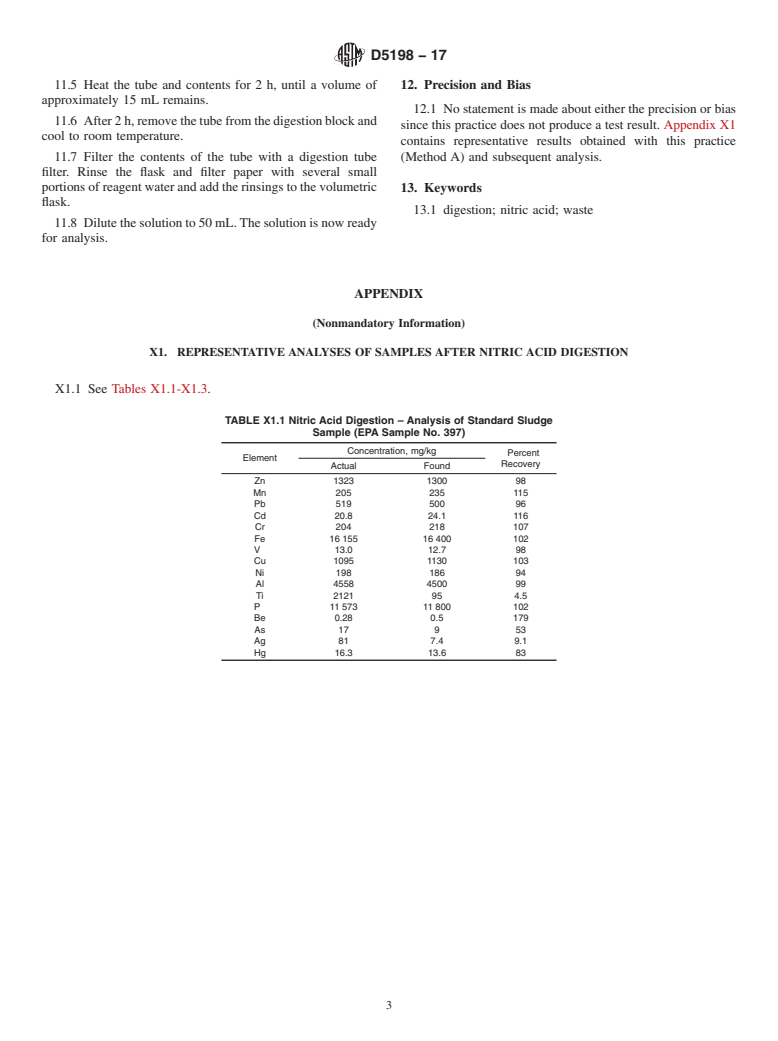
Note 1: This practice has been used successfully for oily sludges and a municipal digested sludge standard [Environmental Protection Agency (EPA) Sample No. 397]. The practice may be applicable to some elements not listed above, such as arsenic, barium, selenium, cobalt, magnesium, and calcium. Refractory elements such as silicon, silver, and titanium, as well as organo-mercury, are not solubilized by this practice.
1.3 This practice has been divided into two methods, A and B, with Method A utilizing an electric hot plate and Method B utilizing an electric digestion block.
1.4 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.5 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of regulatory limitations prior to use.
1.6 This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
General Information
Relations
Buy Standard
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
Designation: D5198 − 17
Standard Practice for
1
Nitric Acid Digestion of Solid Waste
This standard is issued under the fixed designation D5198; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 2. Referenced Documents
2
2.1 ASTM Standards:
1.1 This practice describes the partial digestion of solid
D1193 Specification for Reagent Water
waste using nitric acid for the subsequent determination of the
D5681 Terminology for Waste and Waste Management
total recoverable content of inorganic constituents.
1.2 This practice is to be used when the concentrations of
3. Terminology
total recoverable elements are to be determined from a waste
3.1 Definitions—For definitions of terms used in this
sample. Total recoverable elements are often not equivalent to
standard, refer to Terminology D5681.
total elemental content, because of the solubility of the
speciated forms of the element in the sample matrix. Recovery
4. Summary of Practice
from refractory sample matrices, such as soils, is usually
4.1 A weighed portion of the waste sample is mixed with
significantly less than total concentrations of the elements
1 + 1 nitric acid (HNO ) in an Erlenmeyer flask. The flask is
present.
3
heated on an electric hot plate (MethodA) or electric digestion
NOTE 1—This practice has been used successfully for oily sludges and
block (Method B) for2hat90to95°Cto dissolve the
a municipal digested sludge standard [Environmental Protection Agency
elements of interest.After cooling, the contents of the flask are
(EPA) Sample No. 397].The practice may be applicable to some elements
diluted with reagent water and filtered, and the filtrate is made
not listed above, such as arsenic, barium, selenium, cobalt, magnesium,
and calcium. Refractory elements such as silicon, silver, and titanium, as up to appropriate volume for subsequent analysis.
well as organo-mercury, are not solubilized by this practice.
5. Significance and Use
1.3 This practice has been divided into two methods,Aand
B, with MethodAutilizing an electric hot plate and Method B 5.1 Aknowledge of the inorganic composition of a waste is
utilizing an electric digestion block.
often required for the selection of appropriate waste disposal
practices. Solid waste may exist in a variety of forms and
1.4 The values stated in SI units are to be regarded as
contain a range of organic and inorganic constituents. This
standard. No other units of measurement are included in this
practice describes a digestion procedure which dissolves many
standard.
of the toxic inorganic constituents and produces a solution
1.5 This standard does not purport to address all of the
suitable for determination of total recoverable contents by such
safety concerns, if any, associated with its use. It is the
techniques as atomic absorption spectroscopy, atomic emission
responsibility of the user of this standard to establish appro-
spectroscopy, and so forth. The relatively large sample size
priate safety, health, and environmental practices and deter-
aids representative sampling of heterogenous wastes. The
mine the applicability of regulatory limitations prior to use.
relatively small dilution factor allows lower detection limits
1.6 This international standard was developed in accor- than most other sample digestion methods. Volatile metals,
dance with internationally recognized principles on standard-
such as lead and mercury, are not lost during this digestion
ization established in the Decision on Principles for the procedure, however organo-lead and organo-mercury may not
Development of International Standards, Guides and Recom-
be completely digested. Hydride-forming elements, such as
mendations issued by the World Trade Organization Technical arsenic and selenium, may be partially lost. Samples with total
Barriers to Trade (TBT) Committee.
metal contents greater than 5 % may give low results. The
analyst is responsible for determining whether this practice is
applicable to the solid waste being tested.
1
This practice is under the jurisdiction of ASTM Committee D34 on Waste
Management and is the direct responsibility of Subcommittee D34.01.06 on
2
Analytical Methods. For referenced ASTM standards, visit the ASTM website, www.astm.org, or
Current edition approved Dec. 1, 2017. Published December 2017. Originally contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
approved in 1992. Last previous edition approved in 2009 as D519
...
This document is not an ASTM standard and is intended only to provide the user of an ASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
Designation: D5198 − 09 D5198 − 17
Standard Practice for
1
Nitric Acid Digestion of Solid Waste
This standard is issued under the fixed designation D5198; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope
1.1 This practice describes the partial digestion of solid waste using nitric acid for the subsequent determination of inorganic
constituents by plasma emission spectroscopy or atomic absorption spectroscopy.the total recoverable content of inorganic
constituents.
1.2 The following elements may be solubilized by this practice:
aluminum manganese
beryllium mercury
cadmium nickel
chromium phosphorus
copper vanadium
iron zinc
lead
1.2 This practice is to be used when the concentrations of total recoverable elements are to be determined from a waste sample.
Total recoverable elements may or may not be are often not equivalent to total elements, depending on the element sought and
elemental content, because of the solubility of the speciated forms of the element in the sample matrix. Recovery from refractory
sample matrices, such as soils, is usually significantly less than total concentrations of the elements present.
NOTE 1—This practice has been used successfully for oily sludges and a municipal digested sludge standard [Environmental Protection Agency (EPA)
Sample No. 397]. The practice may be applicable to some elements not listed above, such as arsenic, barium, selenium, cobalt, magnesium, and calcium.
Refractory elements such as silicon, silver, and titanium, as well as organo-mercuryorgano-mercury, are not solubilized by this practice.
1.3 This practice has been divided into two methods, A and B, to account for the advent of digestion blocks. Method A
utilizeswith Method A utilizing an electric hot plate; plate and Method B utilizesutilizing an electric digestion block.
1.4 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.5 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility
of the user of this standard to establish appropriate safety safety, health, and healthenvironmental practices and determine the
applicability of regulatory limitations prior to use.For specific hazard statements, see Section 7.
1.6 This international standard was developed in accordance with internationally recognized principles on standardization
established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued
by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
2. Referenced Documents
2
2.1 ASTM Standards:
D1193 Specification for Reagent Water
D5681 Terminology for Waste and Waste Management
3. Terminology
3.1 Definitions—For definitions of terms used in this standard, refer to Terminology D5681.
1
This practice is under the jurisdiction of ASTM Committee D34 on Waste Management and is the direct responsibility of Subcommittee D34.01.06 on Analytical
Methods.
Current edition approved Feb. 1, 2009Dec. 1, 2017. Published March 2009December 2017. Originally approved in 1992. Last previous edition approved in 20032009 as
D5198 – 92 (2003).D5198 – 09. DOI: 10.1520/D5198-09.10.1520/D5198-17.
2
For referenced ASTM standards, visit the ASTM website, www.astm.org, or contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM Standards
volume information, refer to the standard’s Document Summary page on the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
D5198 − 17
4. Summary of Practice
4.1 A weighed portion of the waste sample is mixed with 1 + 1 nitric acid (HNO ) in an Erlenmeyer flask. The flask is heated
3
on an electric hot plate (Method A) or electric digestion block (Method B) for 2 h at 90 to 95°C95 °C to dissolve the elements of
interest. After cooling, the contents of the flask are diluted with reagent water and filtered, and the filtrate is made up to appropriate
volume for subsequent analysis.
5. Significance and Use
5.1 A knowledge of the inorganic composition of a waste is often required for the selection of appropriate waste disposal
practices. Solid waste ma
...










Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.