ASTM D2650-10
(Test Method)Standard Test Method for Chemical Composition of Gases By Mass Spectrometry
Standard Test Method for Chemical Composition of Gases By Mass Spectrometry
SIGNIFICANCE AND USE
A knowledge of the composition of refinery gases is useful in diagnosing the source of plant upsets, in determining the suitability of certain gas streams for use as fuel, or as feedstocks for polymerization and alkylation, and for monitoring the quality of commercial gases.
SCOPE
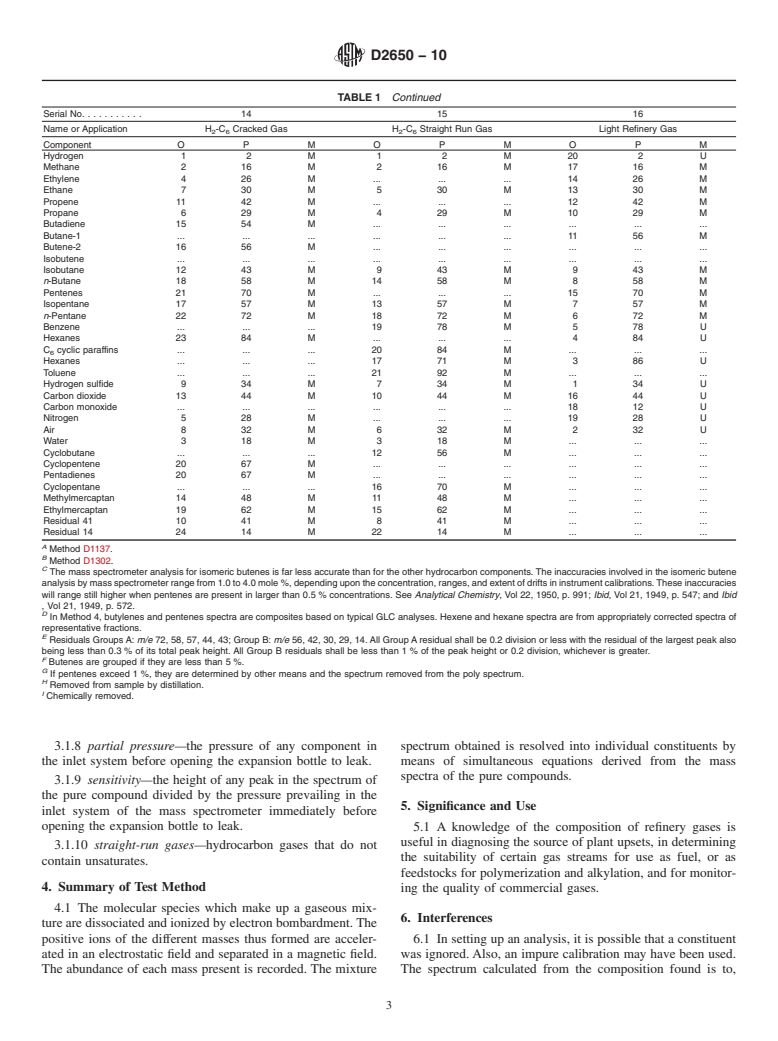
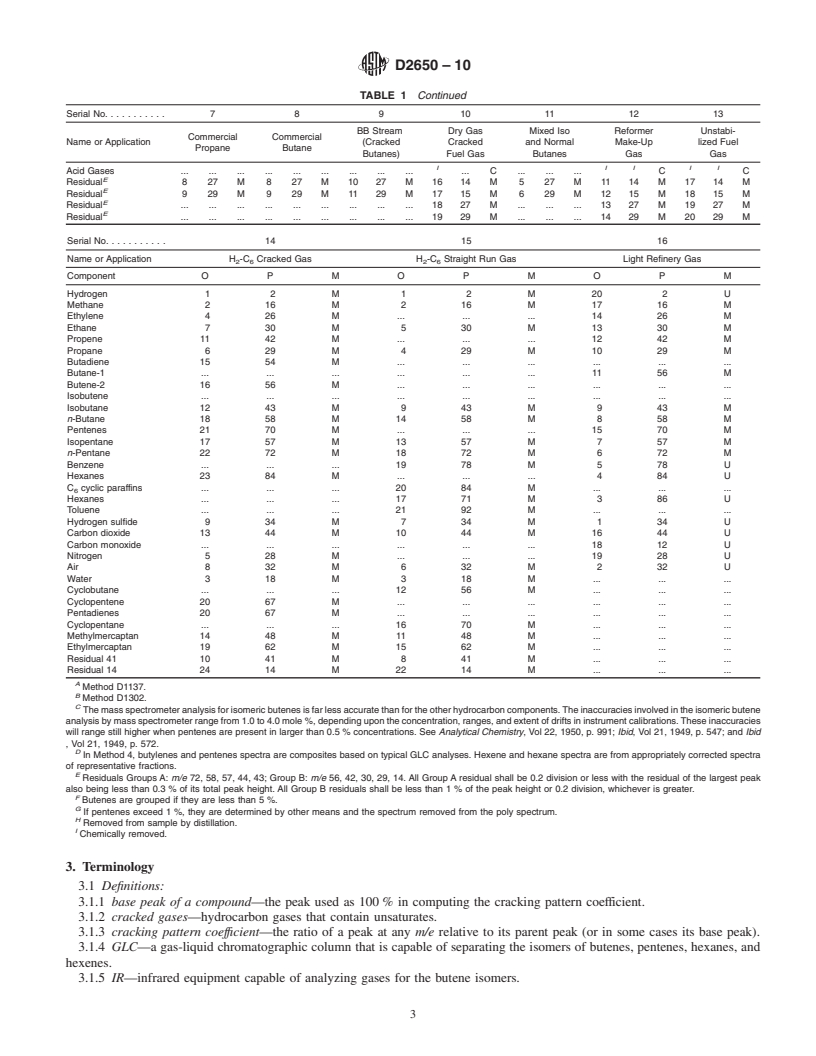
1.1 This test method covers the quantitative analysis of gases containing specific combinations of the following components: hydrogen; hydrocarbons with up to six carbon atoms per molecule; carbon monoxide; carbon dioxide; mercaptans with one or two carbon atoms per molecule; hydrogen sulfide; and air (nitrogen, oxygen, and argon). This test method cannot be used for the determination of constituents present in amounts less than 0.1 mole %. Dimethylbutanes are assumed absent unless specifically sought. Note 1Although experimental procedures described herein are uniform, calculation procedures vary with application. The following influences guide the selection of a particular calculation: qualitative mixture composition; minimum error due to components presumed absent; minimum cross interference between known components; maximum sensitivity to known components; low frequency and complexity of calibration; and type of computing machinery.
Because of these influences, a tabulation of calculation procedures recommended for stated applications is presented in Section ().Note 2
This test method was developed on Consolidated Electrodynamics Corporation Type 103 Mass Spectrometers. Users of other instruments may have to modify operating parameters and the calibration procedure.
This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Buy Standard
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: D2650 − 10
StandardTest Method for
1
Chemical Composition of Gases by Mass Spectrometry
This standard is issued under the fixed designation D2650; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope* Types of Gaseous Mixtures by the Mass Spectrometer
3
(Withdrawn 1981)
1.1 This test method covers the quantitative analysis of
D1247 Test Method for Sampling Manufactured Gas (With-
gases containing specific combinations of the following com-
3
drawn 1986)
ponents: hydrogen; hydrocarbons with up to six carbon atoms
D1265 Practice for Sampling Liquefied Petroleum (LP)
per molecule; carbon monoxide; carbon dioxide; mercaptans
Gases, Manual Method
with one or two carbon atoms per molecule; hydrogen sulfide;
D1302 Test Method for Analysis of Carbureted Water Gas
and air (nitrogen, oxygen, and argon). This test method cannot
3
by the Mass Spectrometer (Withdrawn 1967)
be used for the determination of constituents present in
4
2.2 American Petroleum Institute Standards:
amounts less than 0.1 mole %. Dimethylbutanes are assumed
MPMS 14.1 Collecting and Handling of Natural Gas
absent unless specifically sought.
Samples for Custody Transfer
NOTE 1—Although experimental procedures described herein are
5
2.3 Gas Producers Association Standards:
uniform, calculation procedures vary with application. The following
influences guide the selection of a particular calculation: qualitative GPA 2166 Obtaining Natural Gas Samples for Analysis by
mixture composition; minimum error due to components presumed
Gas Chromatography
absent; minimum cross interference between known components; maxi-
mum sensitivity to known components; low frequency and complexity of
3. Terminology
calibration; and type of computing machinery.
Because of these influences, a tabulation of calculation procedures
3.1 Definitions:
recommended for stated applications is presented in Section 12 (Table 1).
3.1.1 base peak of a compound—the peak used as 100 % in
NOTE 2—This test method was developed on Consolidated Electrody-
computing the cracking pattern coefficient.
namics Corporation Type 103 Mass Spectrometers. Users of other
instruments may have to modify operating parameters and the calibration
3.1.2 cracked gases—hydrocarbon gases that contain un-
procedure.
saturates.
1.2 The values stated in SI units are to be regarded as
3.1.3 cracking pattern coeffıcient—the ratio of a peak at any
standard. No other units of measurement are included in this
m/e relative to its parent peak (or in some cases its base peak).
standard.
3.1.4 GLC—a gas-liquid chromatographic column that is
1.3 This standard does not purport to address all of the
capable of separating the isomers of butenes, pentenes,
safety concerns, if any, associated with its use. It is the
hexanes, and hexenes.
responsibility of the user of this standard to establish appro-
3.1.5 IR—infraredequipmentcapableofanalyzinggasesfor
priate safety and health practices and determine the applica-
the butene isomers.
bility of regulatory limitations prior to use.
3.1.6 mass number or m/e value of an ion—the quotient of
2. Referenced Documents the mass of that ion (given in atomic mass units) and its
2 positive charge (number of electrons lost during ionization).
2.1 ASTM Standards:
3.1.7 parent peak of a compound—thepeakatwhichthe m/e
D1137 Method for Analysis of Natural Gases and Related
is equal to the sum of the atomic mass values for that
compound. This peak is sometimes used as 100 % in comput-
1
This test method is under the jurisdiction of ASTM Committee D02 on
ing the cracking pattern coefficients.
Petroleum Products, Liquid Fuels, and Lubricants and is the direct responsibility of
Subcommittee D02.04.0M on Mass Spectroscopy.
CurrenteditionapprovedMay1,2010.PublishedJuly2010.Originallyapproved
3
in 1967. Last previous edition approved in 2004 as D2650–04. DOI: 10.1520/ The last approved version of this historical standard is referenced on
D2650-10. www.astm.org.
2 4
For referenced ASTM standards, visit the ASTM website, www.astm.org, or Available from American Petroleum Institute (API), 1220 L. St., NW,
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM Washington, DC 20005-4070, http://www.api.org.
5
Standards volume information, refer to the standard’s Document Summary page on Available from Gas ProcessorsAssociation (GPA), 6526 E. 60th St., Tulsa, OK
the ASTM website. 74145, www.gpaglobal.org.
*A Summary of Changes section appears at the end of this standard
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. Un
...
This document is not anASTM standard and is intended only to provide the user of anASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
Designation:D2650–04 Designation:D2650–10
Standard Test Method for
1
Chemical Composition of Gases Byby Mass Spectrometry
This standard is issued under the fixed designation D2650; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope*
1.1 This test method covers the quantitative analysis of gases containing specific combinations of the following components:
hydrogen; hydrocarbons with up to six carbon atoms per molecule; carbon monoxide; carbon dioxide; mercaptans with one or two
carbon atoms per molecule; hydrogen sulfide; and air (nitrogen, oxygen, and argon). This test method cannot be used for the
determination of constituents present in amounts less than 0.1 mole %. Dimethylbutanes are assumed absent unless specifically
sought.
NOTE 1—Althoughexperimentalproceduresdescribedhereinareuniform,calculationproceduresvarywithapplication.Thefollowinginfluencesguide
theselectionofaparticularcalculation:qualitativemixturecomposition;minimumerrorduetocomponentspresumedabsent;minimumcrossinterference
betweenknowncomponents;maximumsensitivitytoknowncomponents;lowfrequencyandcomplexityofcalibration;andtypeofcomputingmachinery.
Because of these influences, a tabulation of calculation procedures recommended for stated applications is presented in Section 12 (Table 1).
NOTE 2—This test method was developed on Consolidated Electrodynamics Corporation Type 103 Mass Spectrometers. Users of other instruments
may have to modify operating parameters and the calibration procedure.
1.2
1.2 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility
of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory
limitations prior to use.
2. Referenced Documents
2
2.1 ASTM Standards:
3
D1137 Method forAnalysis of Natural Gases and Related Types of Gaseous Mixtures by the Mass Spectrometer D1145 Test
Method
for
Sam-
pling
Natu-
ral
3
Gas
3
D1247 Method of Sampling Manufactured Gas
D1265 Practice for Sampling Liquefied Petroleum (LP) Gases, Manual Method
3
D1302 Method for Analysis of Carbureted Water Gas by the Mass Spectrometer
4
2.2 American Petroleum Institute Standards:
MPMS 14.1 Collecting and Handling of Natural Gas Samples for Custody Transfer
5
2.3 Gas Producers Association Standards:
GPA 2166 Obtaining Natural Gas Samples for Analysis by Gas Chromatography
1
This test method is under the jurisdiction ofASTM Committee D02 on Petroleum Products and Lubricants and is the direct responsibility of Subcommittee D02.04.0M
on Hydrocarbon Analysis.Mass Spectroscopy.
Current edition approved Nov.May 1, 2004.2010. Published November 2004.July 2010. Originally approved in 1967. Last previous edition approved in 19992004 as
D2650–99.D2650–04. DOI: 10.1520/D2650-104.
2
For referencedASTM standards, visit theASTM website, www.astm.org, or contactASTM Customer Service at service@astm.org. For Annual Book of ASTM Standards
volume information, refer to the standard’s Document Summary page on the ASTM website.
3
Withdrawn. The last approved version of this historical standard is referenced on www.astm.org.
4
Crout,P.D.,“AShortMethodforEvaluatingDeterminantsandSolvingSystemsofLinearEquationswithRealorComplexCoefficients,”MarchantCalculatingMachine
Co., Bulletins MM-182 and 183, ASTBA, September 1941. Dwyer, P. S., Psychometria, Vol 6, 1941, p. 101. Hotelling, H., Am. Math. Stat., Vol 14, 1943, p. 1.
4
Available from American Petroleum Institute (API), 1220 L. St., NW, Washington, DC 20005-4070, http://www.api.org.
5
“Triangular Inverse Method,” Analytical Chemistry, Vol 30, 1959, p. 877.
5
Available from Gas Processors Association (GPA), 6526 E. 60th St., Tulsa, OK 74145, www.gpaglobal.org.
*A Summary of Changes section appears at the end of this standard.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
1
---------------------- Page: 1 ----------------------
D2650–10
TABLE 1 Calculation Procedures for Mass Spectrometer Gas Analysis
NOTE—Coding of calculation procedures is as follows:
O = Order peaks are used in the calculation expressed
...








Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.