ASTM D1945-96(2001)
(Test Method)Standard Test Method for Analysis of Natural Gas by Gas Chromatography
Standard Test Method for Analysis of Natural Gas by Gas Chromatography
SCOPE
1.1 This test method covers the determination of the chemical composition of natural gases and similar gaseous mixtures within the range of composition shown in Table 1. This test method may be abbreviated for the analysis of lean natural gases containing negligible amounts of hexanes and higher hydrocarbons, or for the determination of one or more components, as required.
1.2 The values stated in SI units are to be regarded as the standard. The values given in parentheses are for information only.
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superceded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
Designation: D 1945 – 96 (Reapproved 2001)
Standard Test Method for
Analysis of Natural Gas by Gas Chromatography
This standard is issued under the fixed designation D 1945; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
TABLE 1 Natural Gas Components and Range of
1. Scope
Composition Covered
1.1 This test method covers the determination of the chemi-
Component Mol %
cal composition of natural gases and similar gaseous mixtures
Helium 0.01 to 10
within the range of composition shown in Table 1. This test
Hydrogen 0.01 to 10
method may be abbreviated for the analysis of lean natural
Oxygen 0.01 to 20
Nitrogen 0.01 to 100
gases containing negligible amounts of hexanes and higher
Carbon dioxide 0.01 to 20
hydrocarbons, or for the determination of one or more compo-
Methane 0.01 to 100
nents, as required.
Ethane 0.01 to 100
Hydrogen sulfide 0.3 to 30
1.2 The values stated in SI units are to be regarded as the
Propane 0.01 to 100
standard. The values given in parentheses are for information
Isobutane 0.01 to 10
only.
n-Butane 0.01 to 10
Neopentane 0.01 to 2
1.3 This standard does not purport to address all of the
Isopentane 0.01 to 2
safety concerns, if any, associated with its use. It is the
n-Pentane 0.01 to 2
responsibility of the user of this standard to establish appro-
Hexane isomers 0.01 to 2
Heptanes+ 0.01 to 1
priate safety and health practices and determine the applica-
bility of regulatory limitations prior to use.
2. Referenced Documents
sponding values obtained with the reference standard.
2.1 ASTM Standards:
4. Significance and Use
D 2597 Test Method for Analysis of Demethanized Hydro-
4.1 This test method is of significance for providing data for
carbon Liquid Mixtures Containing Nitrogen and Carbon
calculating physical properties of the sample, such as heating
Dioxide by Gas Chromatography
value and relative density, or for monitoring the concentrations
D 3588 Practice for Calculating Heat Value, Compressibil-
of one or more of the components in a mixture.
ity Factor, and Relative Density (Specific Gravity) of
Gaseous Fuels
5. Apparatus
E 260 Practice for Packed Column Gas Chromatography
5.1 Detector—The detector shall be a thermal-conductivity
type, or its equivalent in sensitivity and stability. The thermal
3. Summary of Test Method
conductivity detector must be sufficiently sensitive to produce
3.1 Components in a representative sample are physically
a signal of at least 0.5 mV for 1 mol % n-butane in a 0.25-mL
separated by gas chromatography (GC) and compared to
sample.
calibration data obtained under identical operating conditions
5.2 Recording Instruments—Either strip-chart recorders or
from a reference standard mixture of known composition. The
electronic integrators, or both, are used to display the separated
numerous heavy-end components of a sample can be grouped
components. Although a strip-chart recorder is not required
into irregular peaks by reversing the direction of the carrier gas
when using electronic integration, it is highly desirable for
through the column at such time as to group the heavy ends
evaluation of instrument performance.
either as C and heavier, C and heavier, or C and heavier. The
5 6 7
5.2.1 The recorder shall be a strip-chart recorder with a
composition of the sample is calculated by comparing either
full-range scale of 5 mV or less (1 mV preferred). The width of
the peak heights, or the peak areas, or both, with the corre-
the chart shall be not less than 150 mm. A maximum pen
response time of2s(1s preferred) and a minimum chart speed
This test method is under the jurisdiction of ASTM Committee D03 on Gaseous
of 10 mm/min shall be required. Faster speeds up to 100
Fuels and is the direct responsibility of Subcommittee D03.07 on Analysis of
mm/min are desirable if the chromatogram is to be interpreted
Chemical Composition of Gaseous Fuels.
Current edition approved Nov. 10, 1996. Published January 1997. Originally
using manual methods to obtain areas.
published as D 1945 – 62 T. Last previous edition D 1945 – 91.
5.2.2 Electronic or Computing Integrators—Proof of sepa-
Annual Book of ASTM Standards, Vol 05.02.
3 ration and response equivalent to that for a recorder is required
Annual Book of ASTM Standards, Vol 05.05.
Annual Book of ASTM Standards, Vol 14.02. for displays other than by chart recorder. Baseline tracking
Copyright © ASTM, 100 Barr Harbor Drive, West Conshohocken, PA 19428-2959, United States.
NOTICE: This standard has either been superceded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
D 1945
with tangent skim peak detection is recommended. 5.6 Detector Temperature Control—Maintain the detector
5.3 Attenuator—If the chromatogram is to be interpreted temperature at a temperature constant to 0.3°C during the
using manual methods, an attenuator must be used with the course of the sample run and the corresponding reference run.
detector output signal to maintain maximum peaks within the The detector temperature shall be equal to or greater than the
recorder chart range. The attenuator must be accurate to within maximum column temperature.
0.5 % between the attenuator range steps. 5.7 Carrier Gas Controls—The instrument shall be
5.4 Sample Inlet System: equipped with suitable facilities to provide a flow of carrier gas
5.4.1 The sample inlet system shall be constructed of through the analyzer and detector at a flow rate that is constant
materials that are inert and nonadsorptive with respect to the to 1 % throughout the analysis of the sample and the reference
components in the sample. The preferred material of construc- standard. The purity of the carrier gas may be improved by
tion is stainless steel. Copper, brass, and other copper-bearing flowing the carrier gas through selective filters prior to its entry
alloys are unacceptable. The sample inlet system from the into the chromatograph.
cylinder valve to the GC column inlet must be maintained at a 5.8 Columns:
temperature constant to 61°C. 5.8.1 The columns shall be constructed of materials that are
5.4.2 Provision must be made to introduce into the carrier inert and nonadsorptive with respect to the components in the
gas ahead of the analyzing column a gas-phase sample that has sample. The preferred material of construction is stainless
been entrapped in a fixed volume loop or tubular section. The steel. Copper and copper-bearing alloys are unacceptable.
fixed loop or section shall be so constructed that the total 5.8.2 An adsorption-type column and a partition-type col-
volume, including dead space, shall not normally exceed 0.5 umn may be used to make the analysis.
mL at 1 atm. If increased accuracy of the hexanes and heavier
NOTE 2—See Practice E 260.
portions of the analysis is required, a larger sample size may be
5.8.2.1 Adsorption Column—This column must completely
used (see Test Method D 2597). The sample volume must be
separate oxygen, nitrogen, and methane. A 13X molecular
reproducible such that successive runs agree within 1 % on
sieve 80/100 mesh is recommended for direct injection. A 5A
each component. A flowing sample inlet system is acceptable
column can be used if a pre-cut column is present to remove
as long as viscosity effects are accounted for.
interfering hydrocarbons. If a recorder is used, the recorder pen
NOTE 1—The sample size limitation of 0.5 mL or smaller is selected
must return to the baseline between each successive peak. The
relative to linearity of detector response, and efficiency of column
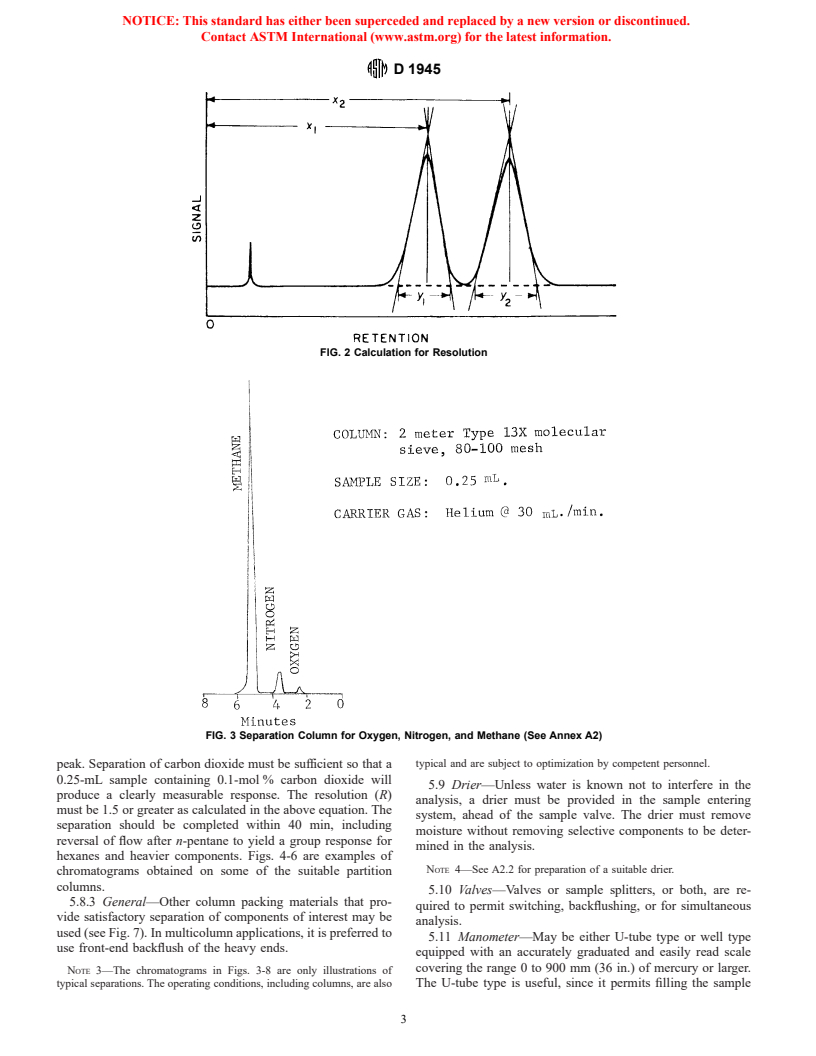
resolution (R) must be 1.5 or greater as calculated in the
separation. Larger samples may be used to determine low-quantity
following equation:
components to increase measurement accuracy.
x 2 x
2 1
5.4.3 An optional manifold arrangement for entering
R~1,2! 5 3 2, (1)
y 1 y
2 1
vacuum samples is shown in Fig. 1.
5.5 Column Temperature Control: where x ,x are the retention times and y ,y are the peak
1 2 1 2
5.5.1 Isothermal—When isothermal operation is used, widths. Fig. 2 illustrates the calculation for resolution. Fig. 3 is
maintain the analyzer columns at a temperature constant to a chromatogram obtained with an adsorption column.
0.3°C during the course of the sample run and corresponding 5.8.2.2 Partition Column—This column must separate
reference run. ethane through pentanes, and carbon dioxide. If a recorder is
5.5.2 Temperature Programming—Temperature program- used, the recorder pen must return to the base line between
ming may be used, as feasible. The oven temperature shall not each peak for propane and succeeding peaks, and to base line
exceed the recommended temperature limit for the materials in within 2 % of full-scale deflection for components eluted ahead
the column. of propane, with measurements being at the attenuation of the
FIG. 1 Suggested Manifold Arrangement for Entering Vacuum Samples
NOTICE: This standard has either been superceded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
D 1945
FIG. 2 Calculation for Resolution
FIG. 3 Separation Column for Oxygen, Nitrogen, and Methane (See Annex A2)
typical and are subject to optimization by competent personnel.
peak. Separation of carbon dioxide must be sufficient so that a
0.25-mL sample containing 0.1-mol % carbon dioxide will
5.9 Drier—Unless water is known not to interfere in the
produce a clearly measurable response. The resolution (R)
analysis, a drier must be provided in the sample entering
must be 1.5 or greater as calculated in the above equation. The
system, ahead of the sample valve. The drier must remove
separation should be completed within 40 min, including
moisture without removing selective components to be deter-
reversal of flow after n-pentane to yield a group response for
mined in the analysis.
hexanes and heavier components. Figs. 4-6 are examples of
NOTE 4—See A2.2 for preparation of a suitable drier.
chromatograms obtained on some of the suitable partition
columns.
5.10 Valves—Valves or sample splitters, or both, are re-
5.8.3 General—Other column packing materials that pro-
quired to permit switching, backflushing, or for simultaneous
vide satisfactory separation of components of interest may be
analysis.
used (see Fig. 7). In multicolumn applications, it is preferred to
5.11 Manometer—May be either U-tube type or well type
use front-end backflush of the heavy ends.
equipped with an accurately graduated and easily read scale
covering the range 0 to 900 mm (36 in.) of mercury or larger.
NOTE 3—The chromatograms in Figs. 3-8 are only illustrations of
typical separations. The operating conditions, including columns, are also The U-tube type is useful, since it permits filling the sample
NOTICE: This standard has either been superceded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
D 1945
FIG. 4 Chromatogram of Natural Gas (BMEE Column) (See Annex A2)
FIG. 5 Chromatogram of Natural Gas (Silicone 200/500 Column) (See Annex A2)
loop with up to two atmospheres of sample pressure, thus 5.12 Vacuum Pump—Must have the capability of producing
extending the range of all components. The well type inher- a vacuum of 1 mm of mercury absolute or less.
ently offers better precision and is preferred when calibrating
6. Preparation of Apparatus
with pure components. Samples with up to one atmosphere of
pressure can be entered. With either type manometer the mm 6.1 Linearity Check—To establish linearity of response for
scale can be read more accurately than the inch scale. Caution the thermal conductivity detector, it is necessary to complete
should be used handling mercury because of its toxic nature. the following procedure:
Avoid contact with the skin as much as possible. Wash 6.1.1 The major component of interest (methane for natural
thoroughly after contact. gas) is charged to the chromatograph by way of the fixed-size
NOTICE: This standard has either been superceded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
D 1945
FIG. 6 Chromatogram of Natural Gas (See Annex A2)
FIG. 7 Chromatogram of Natural Gas (Multi-Column Application) (See Annex A2)
sample loop at partial pressure increments of 13 kPa (100 mm 6.1.4 Any curved line indicates the fixed volume sample
Hg) from 13 to 100 kPa (100 to 760 mm Hg) or the prevailing loop is too large. A smaller loop size should replace the fixed
atmospheric pressure. volume loop and 6.1.1 through 6.1.4 should be repeated (see
6.1.2 The integrated peak responses for the area generated at Fig. 9).
each of the pressure increments are plotted versus their partial 6.1.5 The linearity over the range of interest must be known
pressure (see Fig. 9). for each component. It is useful to construct a table noting the
6.1.3 The plotted results should yield a straight line. A response factor deviation in changing concentration. (See Table
perfectly linear response would display a straight line at a 45° 2 and Table 3).
angle using the logarithmic values. 6.1.6 It should be noted that nitrogen, methane, and ethane
NOTICE: This standard has either been superceded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
D 1945
FIG. 8 Separation of Helium and Hydrogen
exhibit less than 1 % compressibility at atmospheric pressure. 6.2.4 Repeat 6.2.3 for 26, 39, 52, 65, 78, and 91 kPa (200,
Other natural gas components do exhibit a significant com- 300, 400, 500, 600, and 700 mm Hg) on the manometer,
pressibility at pressures less than atmospheric. recording the peak area obtained for sample analysis at each of
6.1.7 Most components that have vapor pressures of less these pressures.
than 100 kPa (15 psia) cannot be used as a pure gas for a
6.2.5 Plot the area data (x axis) versus the partial pressures
linearity study because they will not exhibit sufficient vapor
(y axis) on a linear graph as shown in Fig. 9.
pressure for a manometer reading to 100 kPa (760 mm Hg).
6.2.6 An alternative method is to obtain a blend of all the
For these components, a mixture with nitrogen or methane can
components and charge the sample loop at partial pressure ove
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.