IEC 80601-2-30:2009/COR1:2010
(Corrigendum)Corrigendum 1 - Medical electrical equipment - Part 2-30: Particular requirements for the basic safety and essential performance of automated non-invasive sphygmomanometers
Corrigendum 1 - Medical electrical equipment - Part 2-30: Particular requirements for the basic safety and essential performance of automated non-invasive sphygmomanometers
Corrigendum 1 - Appareils électromédicaux - Partie 2-30: Exigences particulières pour la sécurité de base et les performances essentielles des sphygmomanomètres non invasifs automatiques
General Information
- Status
- Published
- Publication Date
- 13-Jan-2010
- Technical Committee
- SC 62D - Particular medical equipment, software, and systems
- Current Stage
- DELPUB - Deleted Publication
- Start Date
- 22-Mar-2018
- Completion Date
- 13-Feb-2026
Relations
- Effective Date
- 05-Sep-2023
- Effective Date
- 05-Sep-2023
IEC 80601-2-30:2009/COR1:2010 - Corrigendum 1 - Medical electrical equipment - Part 2-30: Particular requirements for the basic safety and essential performance of automated non-invasive sphygmomanometers Released:1/14/2010
Get Certified
Connect with accredited certification bodies for this standard

BSI Group
BSI (British Standards Institution) is the business standards company that helps organizations make excellence a habit.

TÜV Rheinland
TÜV Rheinland is a leading international provider of technical services.

TÜV SÜD
TÜV SÜD is a trusted partner of choice for safety, security and sustainability solutions.
Sponsored listings
Frequently Asked Questions
IEC 80601-2-30:2009/COR1:2010 is a standard published by the International Electrotechnical Commission (IEC). Its full title is "Corrigendum 1 - Medical electrical equipment - Part 2-30: Particular requirements for the basic safety and essential performance of automated non-invasive sphygmomanometers". This standard covers: Corrigendum 1 - Medical electrical equipment - Part 2-30: Particular requirements for the basic safety and essential performance of automated non-invasive sphygmomanometers
Corrigendum 1 - Medical electrical equipment - Part 2-30: Particular requirements for the basic safety and essential performance of automated non-invasive sphygmomanometers
IEC 80601-2-30:2009/COR1:2010 is classified under the following ICS (International Classification for Standards) categories: 11.040.01 - Medical equipment in general. The ICS classification helps identify the subject area and facilitates finding related standards.
IEC 80601-2-30:2009/COR1:2010 has the following relationships with other standards: It is inter standard links to IEC 80601-2-30:2009, IEC 80601-2-30:2018. Understanding these relationships helps ensure you are using the most current and applicable version of the standard.
IEC 80601-2-30:2009/COR1:2010 is available in PDF format for immediate download after purchase. The document can be added to your cart and obtained through the secure checkout process. Digital delivery ensures instant access to the complete standard document.
Standards Content (Sample)
IEC 80601-2-30
(First edition – 2009)
Medical electrical equipment - Part 2-30: Particular requirements for the basic safety
and essential performance of automated non-invasive sphygmomanometers
CORRIGENDUM 1
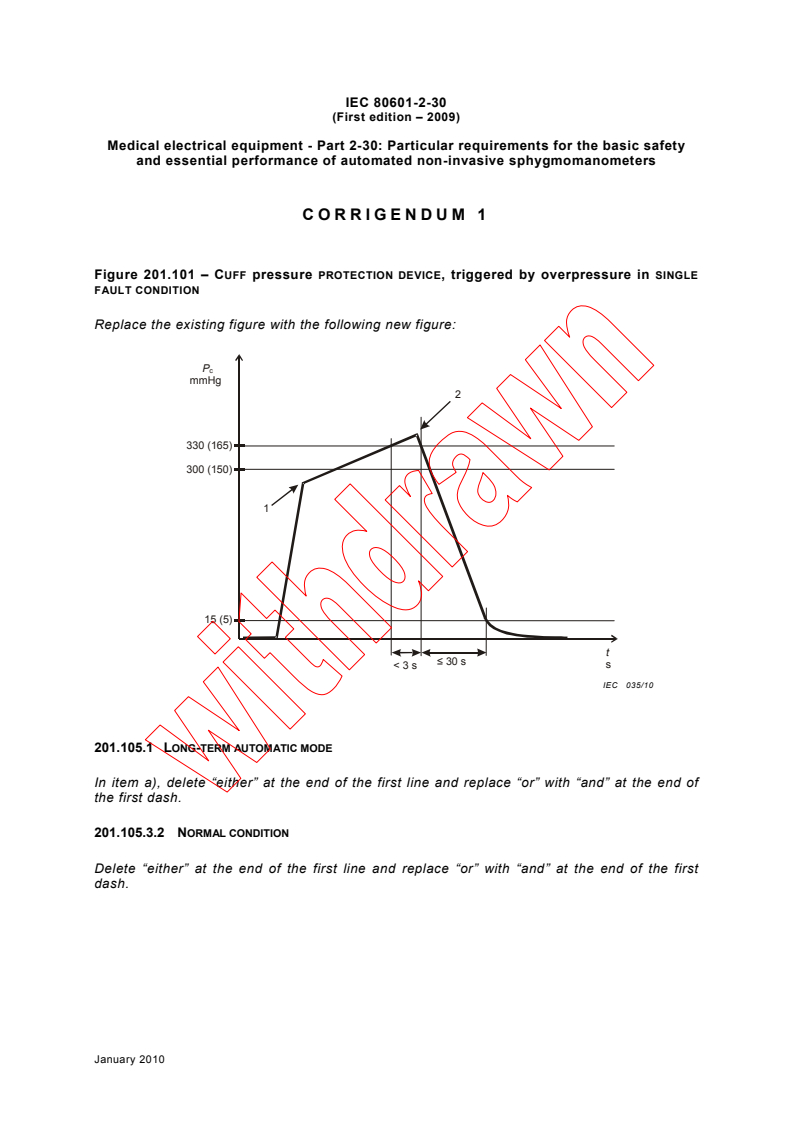
Figure 201.101 – CUFF pressure PROTECTION DEVICE, triggered by overpressure in SINGLE
FAULT CONDITION
Replace the existing figure with the following new figure:
P
c
mmHg
330 (165)
300 (150)
15 (5)
t
≤ 30 s
s
< 3 s
IEC 035/10
ONG-TERM AUTOMATIC MODE
201.105.1 L
In item a), delete “either” at the end of the first line and replace “or” with “and” at the end of
the first dash.
201.105.3.2 NORMAL CONDITION
Delete “either” at the end of the first line and replace “or” with “and” at the end of the first
dash.
January 2010
---------
...



Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.
Loading comments...