ASTM E70-19
(Test Method)Standard Test Method for pH of Aqueous Solutions With the Glass Electrode
Standard Test Method for pH of Aqueous Solutions With the Glass Electrode
SIGNIFICANCE AND USE
4.1 pH is, within the limits described in 1.1, an accurate measurement of the hydrogen ion concentration and thus is widely used for the characterization of aqueous solutions.
4.2 pH measurement is one of the main process control variables in the chemical industry and has a prominent place in pollution control.
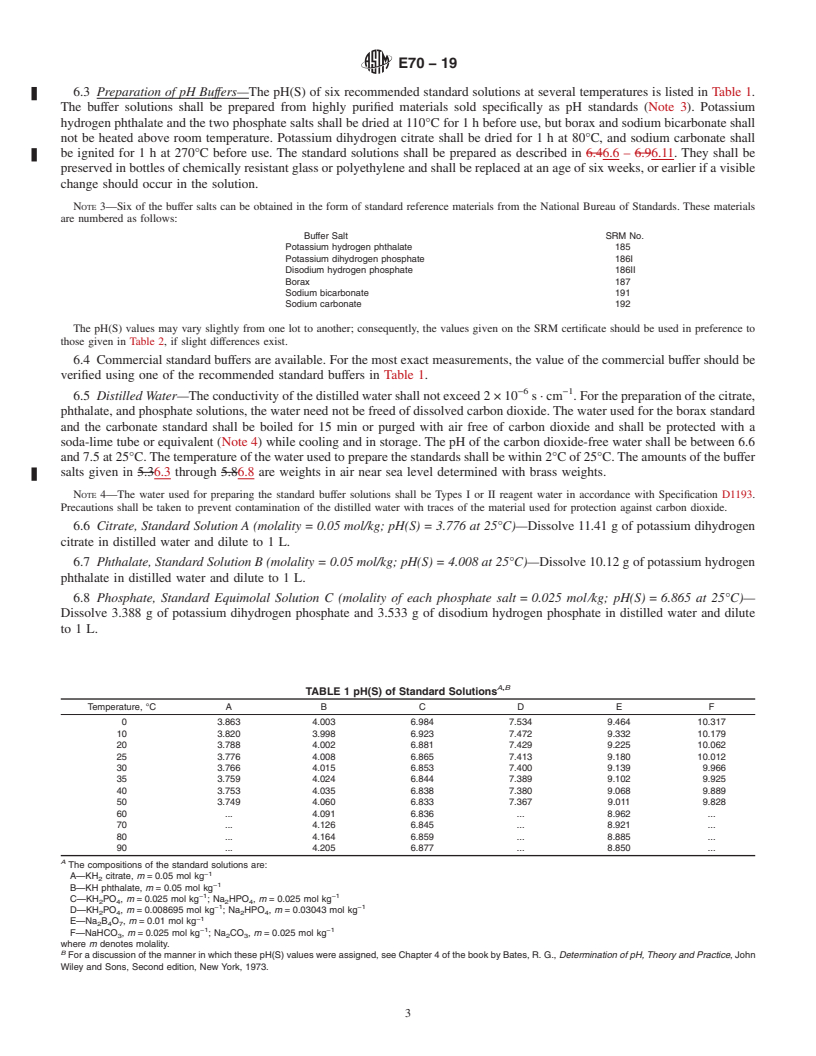
SCOPE
1.1 This test method specifies the apparatus and procedures for the electrometric measurement of pH values of aqueous solutions with the glass electrode. It does not deal with the manner in which the solutions are prepared. pH measurements of good precision can be made in aqueous solutions containing high concentrations of electrolytes or water-soluble organic compounds, or both. It should be understood, however, that pH measurements in such solutions are only a semiquantitative indication of hydrogen ion concentration or activity. The measured pH will yield an accurate result for these quantities only when the composition of the medium matches approximately that of the standard reference solutions. In general, this test method will not give an accurate measure of hydrogen ion activity unless the pH lies between 2 and 12 and the concentration of neither electrolytes nor nonelectrolytes exceeds 0.1 mol/L (M).
1.2 The values stated in SI units are to be regarded as standard. The values in parentheses are for information only.
1.3 In determining the conformance of the test results using this method to applicable specifications, results shall be rounded off in accordance with the rounding-off method of Practice E29.
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of regulatory limitations prior to use.
1.5 This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
General Information
Relations
Buy Standard
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
Designation: E70 − 19
Standard Test Method for
1
pH of Aqueous Solutions With the Glass Electrode
ThisstandardisissuedunderthefixeddesignationE70;thenumberimmediatelyfollowingthedesignationindicatestheyearoforiginal
adoptionor,inthecaseofrevision,theyearoflastrevision.Anumberinparenthesesindicatestheyearoflastreapproval.Asuperscript
epsilon (´) indicates an editorial change since the last revision or reapproval.
This standard has been approved for use by agencies of the U.S. Department of Defense.
1. Scope* 2. Referenced Documents
2
2.1 ASTM Standards:
1.1 This test method specifies the apparatus and procedures
D1193Specification for Reagent Water
for the electrometric measurement of pH values of aqueous
D1293Test Methods for pH of Water
solutions with the glass electrode. It does not deal with the
D5128Test Method for On-Line pH Measurement of Water
manner in which the solutions are prepared. pH measurements
of Low Conductivity
ofgoodprecisioncanbemadeinaqueoussolutionscontaining
D5464Test Method for pH Measurement of Water of Low
high concentrations of electrolytes or water-soluble organic
Conductivity
compounds,orboth.Itshouldbeunderstood,however,thatpH
D6569Test Method for On-Line Measurement of pH
measurements in such solutions are only a semiquantitative
D6809Guide for Quality Control and Quality Assurance
indication of hydrogen ion concentration or activity. The
Procedures for Aromatic Hydrocarbons and Related Ma-
measured pH will yield an accurate result for these quantities
terials
only when the composition of the medium matches approxi-
E29Practice for Using Significant Digits in Test Data to
mately that of the standard reference solutions. In general, this
Determine Conformance with Specifications
test method will not give an accurate measure of hydrogen ion
E180Practice for Determining the Precision of ASTM
activity unless the pH lies between 2 and 12 and the concen-
Methods for Analysis and Testing of Industrial and Spe-
tration of neither electrolytes nor nonelectrolytes exceeds 0.1
3
mol/L (M). cialty Chemicals (Withdrawn 2009)
E691Practice for Conducting an Interlaboratory Study to
1.2 The values stated in SI units are to be regarded as
Determine the Precision of a Test Method
standard. The values in parentheses are for information only.
E1910/E1910MTest Method for Agricultural pH Control
1.3 In determining the conformance of the test results using
Agents, Measurement of pH Change and Buffering Ca-
this method to applicable specifications, results shall be
pacity
rounded off in accordance with the rounding-off method of 4
2.2 Other Documents:
Practice E29.
OSHA Regulations, 29 CFR, paragraphs 1910.1000 and
1.4 This standard does not purport to address all of the 1910.1200
safety concerns, if any, associated with its use. It is the
responsibility of the user of this standard to establish appro- 3. Terminology
priate safety, health, and environmental practices and deter-
3.1 Definitions:
mine the applicability of regulatory limitations prior to use.
3.1.1 pH—defined formally as the negative logarithm to the
1.5 This international standard was developed in accor-
base 10 of the conventional hydrogen ion activity. See Appen-
dance with internationally recognized principles on standard-
dix X1.
ization established in the Decision on Principles for the
3.2 Definitions of Terms Specific to This Standard:
Development of International Standards, Guides and Recom-
mendations issued by the World Trade Organization Technical
Barriers to Trade (TBT) Committee.
2
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Standards volume information, refer to the standard’s Document Summary page on
1
This test method is under the jurisdiction of ASTM Committee D16 on the ASTM website.
3
Aromatic, Industrial, Specialty and Related Chemicals and is the direct responsi- The last approved version of this historical standard is referenced on
bility of Subcommittee D16.04 on Instrumental Analysis. www.astm.org.
4
Current edition approved Nov. 1, 2019. Published December 2019. Originally Available from U.S. Government Printing Office, Superintendent of
approvedin1952.Lastpreviouseditionapprovedin2015asE70–07(2015).DOI: Documents, 732 N. Capitol St., NW, Washington, DC 20401-0001, http://
10.1520/E0070-19. www.access.gpo.gov.
*A Summary of Changes section appears at the end of this standard
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
E70−19
3.2.1 For the purpose of this test method, the term “
...
This document is not an ASTM standard and is intended only to provide the user of an ASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
Designation: E70 − 07 (Reapproved 2015) E70 − 19
Standard Test Method for
1
pH of Aqueous Solutions With the Glass Electrode
This standard is issued under the fixed designation E70; the number immediately following the designation indicates the year of original
adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A superscript
epsilon (´) indicates an editorial change since the last revision or reapproval.
This standard has been approved for use by agencies of the U.S. Department of Defense.
1. Scope*
1.1 This test method specifies the apparatus and procedures for the electrometric measurement of pH values of aqueous
solutions with the glass electrode. It does not deal with the manner in which the solutions are prepared. pH measurements of good
precision can be made in aqueous solutions containing high concentrations of electrolytes or water-soluble organic compounds, or
both. It should be understood, however, that pH measurements in such solutions are only a semiquantitative indication of hydrogen
ion concentration or activity. The measured pH will yield an accurate result for these quantities only when the composition of the
medium matches approximately that of the standard reference solutions. In general, this test method will not give an accurate
measure of hydrogen ion activity unless the pH lies between 2 and 12 and the concentration of neither electrolytes nor
nonelectrolytes exceeds 0.1 mol/L (M).
1.2 The values stated in SI units are to be regarded as standard. The values in parentheses are for information only.
1.3 In determining the conformance of the test results using this method to applicable specifications, results shall be rounded
off in accordance with the rounding-off method of Practice E29.
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility
of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of
regulatory limitations prior to use.
1.5 This international standard was developed in accordance with internationally recognized principles on standardization
established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued
by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
2. Referenced Documents
2
2.1 ASTM Standards:
D1193 Specification for Reagent Water
D1293 Test Methods for pH of Water
D5128 Test Method for On-Line pH Measurement of Water of Low Conductivity
D5464 Test Method for pH Measurement of Water of Low Conductivity
D6569 Test Method for On-Line Measurement of pH
D6809 Guide for Quality Control and Quality Assurance Procedures for Aromatic Hydrocarbons and Related Materials
E29 Practice for Using Significant Digits in Test Data to Determine Conformance with Specifications
E180 Practice for Determining the Precision of ASTM Methods for Analysis and Testing of Industrial and Specialty Chemicals
3
(Withdrawn 2009)
E691 Practice for Conducting an Interlaboratory Study to Determine the Precision of a Test Method
E1910/E1910M Test Method for Agricultural pH Control Agents, Measurement of pH Change and Buffering Capacity
4
2.2 Other Documents:
OSHA Regulations, 29 CFR, paragraphs 1910.1000 and 1910.1200
1
This test method is under the jurisdiction of ASTM Committee D16 on Aromatic, Industrial, Specialty and Related Chemicals and is the direct responsibility of
Subcommittee D16.04 on Instrumental Analysis.
Current edition approved June 1, 2015Nov. 1, 2019. Published June 2015December 2019. Originally approved in 1952. Last previous edition approved in 20072015 as
E70 – 07.E70 – 07 (2015). DOI: 10.1520/E0070-07R15.10.1520/E0070-19.
2
For referenced ASTM standards, visit the ASTM website, www.astm.org, or contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM Standards
volume information, refer to the standard’s Document Summary page on the ASTM website.
3
The last approved version of this historical standard is referenced on www.astm.org.
4
Available from U.S. Government Printing Office, Superintendent of Documents, 732 N. Capitol St., NW, Washington, DC 20401-0001, http://www.access.gpo.gov.
*A Summary of Changes section appears at the end of this standard
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
E70 −
...









Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.