ASTM D6349-21
(Test Method)Standard Test Method for Determination of Major and Minor Elements in Coal, Coke, and Solid Residues from Combustion of Coal and Coke by Inductively Coupled Plasma-Atomic Emission Spectrometry
Standard Test Method for Determination of Major and Minor Elements in Coal, Coke, and Solid Residues from Combustion of Coal and Coke by Inductively Coupled Plasma-Atomic Emission Spectrometry
SIGNIFICANCE AND USE
5.1 A compositional analysis of coal and coke and their associated combustion residues are often useful in assessing their quality. Knowledge of the elemental composition of the associated residues is also useful in predicting the elemental enrichment/depletion compositional behavior of ashes and slags in comparison to the mass fraction in the parent coal. Utilization of the ash by-products and hazardous potential may also depend on the chemical composition and leachability of the inorganic constituents of the coal ash.
5.2 The chemical composition of laboratory-prepared ash may not exactly represent the composition of mineral matter in coal or the composition of fly ash and slag resulting from commerical-scale burning of the coal.
SCOPE
1.1 This test method covers a procedure for the analysis of the commonly determined major and minor elements in coal, coke, and solid residues from combustion of coal and coke. These residues may be laboratory ash, bottom ash, fly ash, flue gas desulfurization sludge, and other combustion process residues.
Note 1: There are two interlaboratory studies associated with this test method. The first was conducted in 1997 ( RR:D05-1035)2 and the second was conducted in 2007 ( RR:D05-1032).3 Sulfur trioxide was only included in the 2007 study, and that study only included combustion residues derived from ash and no combustion residues derived from coke.
1.2 Units-The values stated in SI units are to be regarded as standard. The values given in parentheses after SI units are provided for information only and are not considered standard.
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of regulatory limitations prior to use.
1.4 This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
General Information
Relations
Frequently Asked Questions
ASTM D6349-21 is a standard published by ASTM International. Its full title is "Standard Test Method for Determination of Major and Minor Elements in Coal, Coke, and Solid Residues from Combustion of Coal and Coke by Inductively Coupled Plasma-Atomic Emission Spectrometry". This standard covers: SIGNIFICANCE AND USE 5.1 A compositional analysis of coal and coke and their associated combustion residues are often useful in assessing their quality. Knowledge of the elemental composition of the associated residues is also useful in predicting the elemental enrichment/depletion compositional behavior of ashes and slags in comparison to the mass fraction in the parent coal. Utilization of the ash by-products and hazardous potential may also depend on the chemical composition and leachability of the inorganic constituents of the coal ash. 5.2 The chemical composition of laboratory-prepared ash may not exactly represent the composition of mineral matter in coal or the composition of fly ash and slag resulting from commerical-scale burning of the coal. SCOPE 1.1 This test method covers a procedure for the analysis of the commonly determined major and minor elements in coal, coke, and solid residues from combustion of coal and coke. These residues may be laboratory ash, bottom ash, fly ash, flue gas desulfurization sludge, and other combustion process residues. Note 1: There are two interlaboratory studies associated with this test method. The first was conducted in 1997 ( RR:D05-1035)2 and the second was conducted in 2007 ( RR:D05-1032).3 Sulfur trioxide was only included in the 2007 study, and that study only included combustion residues derived from ash and no combustion residues derived from coke. 1.2 Units-The values stated in SI units are to be regarded as standard. The values given in parentheses after SI units are provided for information only and are not considered standard. 1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of regulatory limitations prior to use. 1.4 This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
SIGNIFICANCE AND USE 5.1 A compositional analysis of coal and coke and their associated combustion residues are often useful in assessing their quality. Knowledge of the elemental composition of the associated residues is also useful in predicting the elemental enrichment/depletion compositional behavior of ashes and slags in comparison to the mass fraction in the parent coal. Utilization of the ash by-products and hazardous potential may also depend on the chemical composition and leachability of the inorganic constituents of the coal ash. 5.2 The chemical composition of laboratory-prepared ash may not exactly represent the composition of mineral matter in coal or the composition of fly ash and slag resulting from commerical-scale burning of the coal. SCOPE 1.1 This test method covers a procedure for the analysis of the commonly determined major and minor elements in coal, coke, and solid residues from combustion of coal and coke. These residues may be laboratory ash, bottom ash, fly ash, flue gas desulfurization sludge, and other combustion process residues. Note 1: There are two interlaboratory studies associated with this test method. The first was conducted in 1997 ( RR:D05-1035)2 and the second was conducted in 2007 ( RR:D05-1032).3 Sulfur trioxide was only included in the 2007 study, and that study only included combustion residues derived from ash and no combustion residues derived from coke. 1.2 Units-The values stated in SI units are to be regarded as standard. The values given in parentheses after SI units are provided for information only and are not considered standard. 1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of regulatory limitations prior to use. 1.4 This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
ASTM D6349-21 is classified under the following ICS (International Classification for Standards) categories: 75.160.10 - Solid fuels. The ICS classification helps identify the subject area and facilitates finding related standards.
ASTM D6349-21 has the following relationships with other standards: It is inter standard links to ASTM D7582-24, ASTM D121-15(2024), ASTM D3180-15(2023), ASTM D7582-15(2023), ASTM D121-15, ASTM D3180-15, ASTM E691-13, ASTM D3174-12, ASTM D3180-12, ASTM E691-11, ASTM D3174-11, ASTM D3173-11, ASTM D3174-04(2010), ASTM D7582-10, ASTM D7582-10e1. Understanding these relationships helps ensure you are using the most current and applicable version of the standard.
You can purchase ASTM D6349-21 directly from iTeh Standards. The document is available in PDF format and is delivered instantly after payment. Add the standard to your cart and complete the secure checkout process. iTeh Standards is an authorized distributor of ASTM standards.
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
Designation: D6349 − 21
Standard Test Method for
Determination of Major and Minor Elements in Coal, Coke,
and Solid Residues from Combustion of Coal and Coke by
Inductively Coupled Plasma—Atomic Emission
Spectrometry
This standard is issued under the fixed designation D6349; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 2. Referenced Documents
2.1 ASTM Standards:
1.1 This test method covers a procedure for the analysis of
D121 Terminology of Coal and Coke
the commonly determined major and minor elements in coal,
D346 Practice for Collection and Preparation of Coke
coke, and solid residues from combustion of coal and coke.
Samples for Laboratory Analysis
These residues may be laboratory ash, bottom ash, fly ash, flue
D1193 Specification for Reagent Water
gas desulfurization sludge, and other combustion process
D2013 Practice for Preparing Coal Samples for Analysis
residues.
D3173 Test Method for Moisture in the Analysis Sample of
NOTE 1—There are two interlaboratory studies associated with this test Coal and Coke
method. The first was conducted in 1997 (RR:D05-1035) and the second
D3174 Test Method forAsh in theAnalysis Sample of Coal
was conducted in 2007 (RR:D05-1032). Sulfur trioxide was only
and Coke from Coal
included in the 2007 study, and that study only included combustion
D3180 Practice for Calculating Coal and Coke Analyses
residues derived from ash and no combustion residues derived from coke.
from As-Determined to Different Bases
1.2 Units—The values stated in SI units are to be regarded
D7348 Test Methods for Loss on Ignition (LOI) of Solid
as standard. The values given in parentheses after SI units are
Combustion Residues
provided for information only and are not considered standard.
D7582 Test Methods for Proximate Analysis of Coal and
Coke by Macro Thermogravimetric Analysis
1.3 This standard does not purport to address all of the
D8146 Guide for Evaluating Test Method Capability and
safety concerns, if any, associated with its use. It is the
Fitness for Use
responsibility of the user of this standard to establish appro-
E691 Practice for Conducting an Interlaboratory Study to
priate safety, health, and environmental practices and deter-
Determine the Precision of a Test Method
mine the applicability of regulatory limitations prior to use.
2.2 ISO Standard:
1.4 This international standard was developed in accor-
ISO/IEC Guide 99:2007 International vocabulary of metrol-
dance with internationally recognized principles on standard-
ogy -- Basic and general concepts and associated terms
ization established in the Decision on Principles for the
(VIM)
Development of International Standards, Guides and Recom-
mendations issued by the World Trade Organization Technical 3. Terminology
Barriers to Trade (TBT) Committee.
3.1 For definitions of terms used in this test method, refer to
Terminology D121.
1 4. Summary of Test Method
This test method is under the jurisdiction of ASTM Committee D05 on Coal
and Coke and is the direct responsibility of Subcommittee D05.29 on Major
4.1 The sample to be analyzed is ashed under standard
Elements in Ash and Trace Elements of Coal.
conditions and ignited to constant mass. The ash is fused with
Current edition approved Sept. 1, 2021. Published October 2021. Originally
approved in 1998. Last previous edition approved in 2013 as D6349 – 13. DOI:
10.1520/D6349-21.
2 4
Supporting data have been filed at ASTM International Headquarters and may For referenced ASTM standards, visit the ASTM website, www.astm.org, or
beobtainedbyrequestingResearchReportRR:D05-1035.ContactASTMCustomer contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Service at service@astm.org. Standards volume information, refer to the standard’s Document Summary page on
Supporting data have been filed at ASTM International Headquarters and may the ASTM website.
beobtainedbyrequestingResearchReportRR:D05-1032.ContactASTMCustomer Available from International Organization for Standardization (ISO), 1 rue de
Service at service@astm.org. Varembé, Case postale 56, CH-1211, Geneva 20, Switzerland, http://www.iso.ch.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
D6349 − 21
TABLE 1 Recommended Wavelengths for Elements Determined
a fluxing agent followed by dissolution of the melt in dilute
by ICP
acid solution.Alternatively, the ash is digested in a mixture of
Element Wavelengths, nm
hydrofluoric, nitric, and hydrochloric acids. The solution is
analyzedbyinductivelycoupledplasma-atomicemissionspec-
Aluminum 396.152, 256.80, 308.215, 309.271
trometry (ICP) for the elements. The basis of the method is the
Barium 455.403, 493.41, 233.53
Calcium 317.93, 315.887, 364.44, 422.67
measurement of atomic emissions. Aqueous solutions of the
Iron 259.940, 271.44, 238.204
samples are nebulized, and a portion of the aerosol that is
Magnesium 279.553, 279.08, 285.21, 277.983
produced is transported to the plasma torch where excitation Manganese 257.610, 294.92, 293.31, 293.93
Phosphorous 178.287, 214.900
and emission occurs. Characteristic line emission spectra are
Potassium 766.491, 769.896
produced by a radio-frequency inductively coupled plasma. A
Silicon 212.412, 288.16, 251. 611
gratingmonochromatorsystemisusedtoseparatetheemission Sodium 588.995, 589.592
Strontium 421.55
lines, and the intensities of the lines are monitored by photo-
Sulfur 182.04
mutilplier tube or photodiode array detection. The photocur-
Titanium 337.280, 350.50, 334.941
rents from the detector are processed and controlled by a
computer system. A background correction technique is re-
quired to compensate for variable background contribution to
that could occur in a sample but for which there is no channel
the determination of elements. Background must be measured
in the instrument array.
adjacent to analyte lines of samples during analysis. The
6.1.2 Table 1 lists the elements determined by this method
positionselectedforthebackgroundintensitymeasurement,on
and the recommended wavelengths using conventional nebu-
eitherorbothsidesoftheanalyticalline,willbedeterminedby
lization. Sulfur may only be determined if the sample is
thecomplexityofthespectrumadjacenttotheanalyteline.The
dissolved by the mixed acid dissolution described in 10.3.2.
position used must be free of spectral interference and reflect
6.1.3 Table 2 lists some interference effects for the recom-
the same change in background intensity as occurs at the
mended wavelengths given in Table 1. The data in Table 2 are
analyte wavelength measured.
intended for use only as a rudimentary guide for the indication
of potential spectral interferences. For this purpose, linear
5. Significance and Use
relations between mass concentration and intensity for the
5.1 A compositional analysis of coal and coke and their
analytes and the interferents can be assumed. The analyst
associated combustion residues are often useful in assessing
should follow the manufacturer’s operating guide to develop
their quality. Knowledge of the elemental composition of the
and apply correction factors to compensate for the interfer-
associated residues is also useful in predicting the elemental
ences.
enrichment/depletion compositional behavior of ashes and
6.1.4 Physical interferences are generally considered to be
slags in comparison to the mass fraction in the parent coal.
effects associated with the sample nebulization and transport
Utilization of the ash by-products and hazardous potential may
processes. Such properties as change in viscosity and surface
also depend on the chemical composition and leachability of
tension can cause significant inaccuracies, especially in
the inorganic constituents of the coal ash.
samples that may contain high dissolved solids or acid
concentrations, or both. The use of a peristaltic pump is
5.2 The chemical composition of laboratory-prepared ash
may not exactly represent the composition of mineral matter in recommended to lessen these interferences. If these types of
interferencesareoperative,theymustbereducedbydilutionof
coal or the composition of fly ash and slag resulting from
the sample or utilization of standard addition techniques, or
commerical-scale burning of the coal.
both. Another problem that can occur from high dissolved
6. Interferences solids is salt buildup at the tip of the nebulizer. This affects
aerosol flow rate causing instrumental drift. Humidifying the
6.1 Several types of interference effects may contribute to
argon before nebulization, the use of a tip washer, or sample
inaccuraciesinthedeterminationofmajorandminorelements.
dilution have been used to control this problem. Also, it has
The analyst should follow the manufacturer’s operating guide
been reported that better control of the argon flow rate,
to develop and apply correction factors to compensate for the
particularly nebulizer flow, improves instrument precision.
interferences. The interferences can be classified as spectral,
This is accomplished with the use of mass flow controllers.
physical, and chemical.
6.1.5 Chemical interferences are characterized by molecular
6.1.1 Spectral interferences can be categorized as overlap of
compound formation, ionization effects, and solute vaporiza-
a spectral line from another element, unresolved overlap of
tioneffects.Normallytheseeffectsarenotpronouncedwiththe
molecular band spectra, background contribution from con-
ICP technique. However, if such effects are observed they can
tinuous or recombination phenomena, and background contri-
be minimized by careful selection of operating conditions
bution from stray light from the line emission of high mass
(such as, incident power, gas flows, observation height, and
concentration elements. The second effect may require selec-
sample uptake rate), by buffering of the sample, matrix
tionofanalternatewavelength.Thethirdandfourtheffectscan
matching, and standard addition procedures. These types of
usuallybecompensatedbyabackgroundcorrectionadjacentto
the analyte line. In addition, users of simultaneous multi-
element instrumentation must assume the responsibility of
Methods for Chemical Analysis of Water and Wastes , (EPA-600/4-79-020),
verifying the absence of spectral interference from an element Metals-4, Method 200.7 CLP-M.
D6349 − 21
TABLE 2 Examples of Analyte Mass Concentration Equivalents (mg/L) Arising from Interference at the 100 mg/L Level
NOTE 1—Dashes indicate that no interference was observed even when interferents were introduced at the following levels: Al, Ca, and Fe =
1000 mg⁄L, Mn = 200 mg⁄L, and Mg = 100 mg⁄L.
Interferents
Analyte Elements Wavelengths, nm Al Ca Fe Mg Mn Ti
Aluminum 308.215 - - - - - - - - - - - - 0.21 - - -
Barium 455.103 - - - - - - - - - - - - - - - - - -
Calcium 317.933 - - - - - - 0.01 0.01 0.04 0.03
Iron 259.940 - - - - - - - - - - - - 0.12 - - -
Magnesium 279.079 - - - 0.02 0.13 - - - 0.25 0.07
Manganese 257.610 0.005 - - - 0.002 0.002 - - - - - -
Silicon 288.148 - - - - - - - - - - - - - - - - - -
Sodium 588.995 - - - - - - - - - - - - - - - 0.08
interferences can be highly dependent on matrix type and the 8. Reagents
specific analyte element.
8.1 Purity of Reagents—Reagent grade chemicals shall be
used in all tests. It is intended that all reagents shall conform to
7. Apparatus
the specifications of the Committee on Analytical Reagents of
7.1 Ashing Furnace, with an adequate air circulation and
the American Chemical Society in which such specifications
capable of having its temperature regulated at 500 °C and
are available. Other grades may be used provided it is first
750 °C.
ascertained that the reagent is of sufficiently high purity to
permit its use without lessening the accuracy of the determi-
7.2 Fusion Furnace, with an operating temperature of
nation.
1000 °C to 1200 °C.
8.2 Purity of Water—Unless otherwise indicated, references
7.3 Meker-Type Burner, with inlets for fuel gas (propane or
natural gas) and compressed air, capable of flame temperatures to water shall be understood to mean Type II reagent water as
defined by Specification D1193.
of 1000 °C to 1200 °C.
7.4 Platinum Dishes or Crucibles,35 mLto85 mLcapacity. 8.3 Standard Stock Solutions—Stock solutions of
1000 mg⁄L for each element are needed for preparation of
Graphite crucibles with 10 mL to 15 mL capacity may also be
used. dilute standards in the range from < 0.1 mg⁄L to 100 mg⁄L.
Prepare standard stock solutions from 99.99 % purity metals or
7.5 Stirring Hotplate and Bars, with operating temperature
salts. Alternatively, one can use commercially available stock
up to 200 °C.
solutions specifically prepared for ICP-AES spectroscopy.
7.6 Polycarbonate Bottles, 250 mL capacity with an O-ring
8.3.1 If multi-element stock standards are prepared, care
seal and screw cap, capable of withstanding temperatures of
should be taken to take into account contamination present for
100 °C to 130 °C, the pressure that is developed during the
otherelementsofinterestineachsingleelementstocksolution.
digestion, and resistant to oxidation. Other types of bottles or
It is recommended that multi-element stocks be purchased
vials may be used provided they are capable of withstanding
fromareputablestandardsvendorandincludeacertificationof
the temperatures and pressures developed during the digestion.
the major element mass concentrations as well as a table of
contaminant elements and mass concentrations.
7.7 Inductively Coupled Plasma-Atomic Emission Spec-
trometer (ICP), either a sequential or simultaneous spectrom-
8.4 Internal Standard Solution—Stock solution of
eter is suitable. Because of the differences between various
1000 mg⁄Lofyttrium(Y),scandium(Sc),indium(In),orother
makes and models of satisfactory instruments, no detailed
suitable element not found in significant mass fractions in the
operating instructions can be provided. Instead, the analyst
test samples.
should follow the instructions provided by the manufacturer of
8.5 Acids:
the particular instrument. Sensitivity, instrumental detection
8.5.1 Hydrochloric Acid—Concentrated hydrochloric acid,
limit, precision, linear dynamic range, and interference effects
12 N, specific gravity 1.19.
must be investigated and established for each individual
8.5.2 Hydrofluoric Acid—Concentrated hydrofluoric acid,
analyte line on that particular instrument. All measurements
29 N, specific gravity 1.17.
must be within the instrument’s linear range in which correc-
8.5.3 Nitric Acid—Concentrated nitric acid, 16 N, specific
tion factors are valid. It is the responsibility of the analyst to
gravity 1.42.
verify that the instrument configuration and operating condi-
8.5.4 Nitric Acid (5 + 95)—Dilute 50 mL of concentrated
tions used satisfy the analytical requirements of this method
nitric acid to 1000 mL.
and to maintain quality control data confirming instrument
performance and analytical results.
Reagent Chemicals, American Chemical Society Specifications, American
NOTE 2—The abbreviation ICPAES is used throughout this test method
Chemical Society, Washington, DC. For suggestions on the testing of reagents not
to refer to Inductively Coupled Plasma Atomic Emission Spectrometry,
listed by the American Chemical Society, see Analar Standards for Laboratory
and it is understood that some manufacturers will instead use the
Chemicals, BDH Ltd., Poole, Dorset, U.K., and the United States Pharmacopeia
abbreviation ICPOES. In all cases, it is understood that ICPAES and and National Formulary, U.S. Pharmacopeial Convention, Inc. (USPC), Rockville,
ICPOES refer to the same technique. MD.
D6349 − 21
8.5.5 Mixed Acid Solution, 70/30 HCl/HF—Mix seven parts 8.12 Initial Calibration Verification Standard(s):
concentrated hydrochloric acid and three parts concentrated 8.12.1 Where possible the initial calibration verification
hydrofluoric acid. standard(s) shall be from alternate producers or different lot
numbers from the calibration standard(s).
8.6 Fluxing Agents— Lithium tetraborate, Li B O , or mix-
2 4 7
8.12.2 Where possible the initial calibration verification
tures of lithium tetraborate (Li B O ) and anhydrous lithium
2 4 7
standard(s) shall be traceable to a primary standard such as a
metaborate (LiBO ).
National Institute of Technology Standard Reference Material
8.7 Boric Acids Solution—1.5 %.
(NIST SRM).
8.8 Hydrogen Peroxide—30 %.
8.13 Periodic Calibration Verification Standard(s)—The
source of these materials can be the same as the calibration
8.9 Wetting Agents—Approximately 0.1 g of reagent grade
materials.
lithium iodide (LiI) or other suitable wetting agent may be
added to the flux to facilitate pooling of the melt and removal 8.14 Primary Control Sample—Amaterial that is processed
of the melt of cooled pellet. following the same procedure as an analytical sample and is a
measurement standard whose quantity value and measurement
8.10 Standard Solution Diluent—Use either 8.10.1 or
uncertainty are established without relation to another mea-
8.10.2.
surementstandardforaquantityofthesamekind(seeISO/IEC
8.10.1 Transfer 4 g, determined to the nearest 0.0001 g, of
Guide 99:2007 International Vocabulary of Basic and General
fluxing agent (see 8.6) into a clean 1000 mLbeaker containing
Terms in Metrology).
a magnetic stirring bar. Add 500 mL of5+95 nitric acid (see
8.15 Secondary Control Sample—A material that is pro-
8.5.4) to the beaker and place on a stirring hot plate. Heat the
cessed following the same procedure as an analytical sample
mixture to just below boiling and maintain this temperature
and is a measurement standard whose quantity value and
with constant stirring until the fluxing agent dissolves. This
measurement uncertainty are assigned through calibration
dissolution process should take about 30 min or less (see Note
against, or comparison with, a primary measurement standard
3). Quantitatively transfer the warm solution to a 1000 mL
for a quantity of the same kind (see ISO/IEC Guide 99:2007
volumetric flask.After the solution cools to room temperature,
International Vocabulary of Basic and General Terms in Me-
dilute to 1000 mL with reagent grade water.
trology).
8.10.2 Transfer 4 g, determined to the nearest 0.0001 g, of
fluxing agent (see 8.6) into a platinum dish (or crucible). Heat
9. Sample Preparation
to 1000 °C to form a liquid and cool. Carefully rinse the
9.1 Coal and Coke—Prepare the analysis sample in accor-
bottom and outside of the platinum dish to remove possible
dance with Practice D2013 for coal or Practice D346 for coke
contamination. Place the cooled platinum dish containing the
by pulverizing the material to pass a 250 µm (No. 60) U.S.A.
flux and a magnetic stirring bar into a clean 1000 mL beaker.
standard sieve.
Add500mLof5+95 nitric acid (see 8.5.4) to the beaker and
9.1.1 Analyze separate test portions for moisture and ash
place immediately on the stirring hotplate. Heat the mixture to
contents in accordance with Test Methods D3173, D3174,or
just below the boiling temperature and maintain this tempera-
D7582 so that calculation to other bases can be made.
ture with constant stirring until the melt dissolves. This
dissolution process should take about 30 min (see Note 3). 9.2 Laboratory Ashing of Coal and Coke Analysis Sample—
After dissolution, remove the platinum dish after rinsing with Prepare the ash from a thoroughly mixed analysis sample of
reagent water and collecting the washings in the acid solution. coal or coke (see 9.1). Spread the coal and coke in a layer not
Quantitatively transfer the warm solution to a 1000 mL volu- over 6 mm in depth in a porcelain, quartz, or fused silica
metric flask. After the solution cools to room temperature, roasting dish. Place the dish in a cold muffle furnace and heat
dilute to 1000 mL with reagent grade water. gradually so that the temperature reaches 500 °C 6 10 °C at
the end of 1 h. Continue the gradual heating until the
NOTE 3—This time and temperature are sufficient to dissolve the melt
temperature rises from 500 °C 6 10 °C to 750 °C 6 15 °C at
completely. If stirring is not maintained constantly, some of the material
the end of 1 h. Maintain the 750 °C temperature until the test
may not dissolve, and the final solution must be filtered before use.
specimen reaches a constant mass or for an additional 2 h.
8.11 Blank Solutions—All of the test methods in this stan-
Allow the dish to cool, transfer to an agate mortar, and grind to
dard require two types of blank solutions. A calibration blank
pass a 75 µm (No. 200) U.S.A. standard sieve. Reignite the ash
that is used to establish the analytical calibration curve and a
at 750 °C for 1 h, allow to cool to room temperature, and
method blank which is used to evaluate possible contamination
determine the mass of portions for analysis.
and assess spectral background. The calibration blank is also
9.3 Solid Combustion Residue—Dry a representative por-
used initially and periodically to verify the baseline of the
tion of the solid residue to constant mass at 107 °C 6 3 °C.
calibration has not changed significantly.
Determine the moisture loss during this drying step if it is
8.11.1 Calibration Blank—The same solution as the Stan-
desirable to calculate results to an as-received basis. Crush the
dard Solution Diluent.
dried portion of the sample to pass a 75 µm (No. 200) U.S.A
8.11.2 Method Blank—The method blank shall be processed
standard sieve. Use a mill that minimizes metal contamination.
through the same digestion procedure as the samples and
consist of all the reagents in the same volumes as used in 9.4 Ashing Solid Combustion Residue—Spread an appropri-
preparing the samples. ate amount of the prepared sample in a layer not over 2 mm in
D6349 − 21
a porcelain, quartz, or fused silica roasting dish. Place the dish ash. Remove the tray with the dish and cool to room tempera-
in a cold muffle furnace and heat gradually so that the ture. Carefully rinse the bottom and outside of the platinum
temperature reaches 500 °C 6 10 °C at the end of 1 h. dish to remove possible contamination; then place in a clean
Continue the gradual heating until the temperature rises from 250 mL or 400 mL beaker. Place a clean TFE-fluorocarbon
500 °C 6 10 °C to 750 °C 6 15 °C at the end of 1 h. Maintain coated magnetic stirring bar in the platinum dish and add
the 750 °C temperature until the combustion residue reaches a 50 mL of (5 + 95) nitric acid (see 8.5.4) to the melt in the
constant mass or for an additional 2 h. Cool the test specimen, platinum dish. Immediately place the beaker with the dish on
grind to pass a 75 µm (No. 200) U.S.A standard sieve, and the stirring hotplate. Stir and heat the solution to just below
reignite at 750 °C for 1 h. boiling and maintain this near boiling condition until the melt
is dissolved or for not more than 30 min. Constantly stir and
9.5 If previously-ignited samples are stored and the absorp-
heat the solution, and repeat the analysis if precipitate is
tionofmoisture,orCO ,orboth,isinquestion,reignitetheash
formed (see Note 5). Remove the platinum dish from the
at 750 °C before use. Alternatively, determine loss on ignition
beaker, rinse the dish with small amounts of reagent water, and
using Test Method D7348 on a separate sample whose mass is
quantitatively transfer the solution to a 100 mL volumetric
determined at the same time as the test portion and make the
flask. Add 1 mL of internal standard to the flask and dilute to
necessary corrections. Thoroughly mix each sample before
the 100 mL mark with water. This solution is 1000 mg⁄L with
weighing.
respect to the total sample and contains 4 g⁄Lof fluxing agent.
Prepare a method blank using the above procedure.
10. Procedure
NOTE 4—Graphite crucibles may be used instead of platinum for the
10.1 The solutions and proportions described below are the
fusion. The graphite crucibles are not to be immersed in the digestion
typical ash samples as represented by American coals.
solution. Pour the red-hot melt directly from the crucible into the acid
Therefore, stronger or weaker dilutions may be required to
solution and proceed with stirring and heating as written above.
establish suitable mass concentrations for those elements NOTE 5—If the stirring is not constantly maintained, some of the
constituentsmayprecipitate,primarilysilicicacid,asaresultofheatingin
varying outside the range of the typical sample.Analysts must
the highly acidic solution.
determinethesensitivityandlinearrangeofcalibrationoftheir
10.3.1.2 If a flame is used for heating, rotate the platinum
own equipment and choose mass concentration ranges for
dish in the flame until a clear melt is obtained. If automated
standards compatible with the samples and instrument specific
fusion equipment is being used, follow the manufacturer’s
to their own work.
programmed steps. If the crucible is inserted manually into the
10.2 To minimize the potential of contamination, platinum
flame using platinum-tipped tongs, stir by swirling for at least
ware must be prepared by boiling in dilute (5 + 95) and rinsing
5 min. When a clear melt is obtained, either pour the hot melt
thoroughlywithreagent-gradewater.Afterthisinitialcleaning,
into 50 mL of (5 + 95) nitric acid (see 8.5.4) in a clean
the platinum ware must be handled with clean tongs and
250 mg⁄L or 400 mL beaker containing a TFE-fluorocarbon
protected from contamination from tabletops, and so forth.All
magnetic stirring bar or cool the crucible and transfer the solid
glassware used in analyses must be equally clean and pro-
pellettothissolution.Ifitcannotbecompletelytransferred,the
tected.
sample should be discarded and re-prepared. Immediately
10.3 Ash Dissolution—Two methods of dissolving the ash
place the beaker on a stirring hot plate. Stir and heat the
samplesareofferedforthistestmethod:fusionandmixedacid. solution to just below boiling and maintain the near boiling
The analyst may choose the method most appropriate for their
condition until the pellet is dissolved or for not more than
laboratory and instrumentation. Laboratories using the fusion 30 min. Constantly stir and heat the solution, and repeat the
method (see 10.3.1) for dissolving the ash should be aware that
analysis if precipitate is formed (see Note 5). Transfer the
a considerable amount of sulfur may be lost from the ash
solutionquantitativelytoa100 mLvolumetricflask.Add1 mL
during the fusion process. A blank test solution containing the of internal standard to the flask and dilute to the 100 mL mark
same mass concentration of reagents used for the ash samples
with water. This solution is 1000 mg/Lwith respect to the total
shall be prepared and analyzed
...
This document is not an ASTM standard and is intended only to provide the user of an ASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
Designation: D6349 − 13 D6349 − 21
Standard Test Method for
Determination of Major and Minor Elements in Coal, Coke,
and Solid Residues from Combustion of Coal and Coke by
Inductively Coupled Plasma—Atomic Emission
Spectrometry
This standard is issued under the fixed designation D6349; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope
1.1 This test method covers a procedure for the analysis of the commonly determined major and minor elements in coal, coke,
and solid residues from combustion of coal and coke. These residues may be laboratory ash, bottom ash, fly ash, flue gas
desulfurization sludge, and other combustion process residues.
NOTE 1—There are two interlaboratory studies associated with this test method. The first was conducted in 1997 (RR:D05-1035) and the second was
conducted in 2007 (RR:D05-1032). Sulfur trioxide was only included in the 2007 study, and that study only included combustion residues derived from
ash and no combustion residues derived from coke.
1.2 Units—The values stated in SI units are to be regarded as standard. No other units of measurement are included in this The
values given in parentheses after SI units are provided for information only and are not considered standard.
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility
of the user of this standard to establish appropriate safety safety, health, and healthenvironmental practices and determine the
applicability of regulatory limitations prior to use.
1.4 This international standard was developed in accordance with internationally recognized principles on standardization
established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued
by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
2. Referenced Documents
2.1 ASTM Standards:
D121 Terminology of Coal and Coke
D346 Practice for Collection and Preparation of Coke Samples for Laboratory Analysis
D1193 Specification for Reagent Water
D2013 Practice for Preparing Coal Samples for Analysis
This test method is under the jurisdiction of ASTM Committee D05 on Coal and Coke and is the direct responsibility of Subcommittee D05.29 on Major Elements in
Ash and Trace Elements of Coal.
Current edition approved Oct. 1, 2013Sept. 1, 2021. Published October 2013October 2021. Originally approved in 1998. Last previous edition approved in 20092013 as
D6349 - 09.D6349 – 13. DOI: 10.1520/D6349-13.10.1520/D6349-21.
Supporting data have been filed at ASTM International Headquarters and may be obtained by requesting Research Report RR:D05-1035. Contact ASTM Customer
Service at service@astm.org.
Supporting data have been filed at ASTM International Headquarters and may be obtained by requesting Research Report RR:D05-1032. Contact ASTM Customer
Service at service@astm.org.
For referenced ASTM standards, visit the ASTM website, www.astm.org, or contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM Standards
volume information, refer to the standard’s Document Summary page on the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
D6349 − 21
D3173 Test Method for Moisture in the Analysis Sample of Coal and Coke
D3174 Test Method for Ash in the Analysis Sample of Coal and Coke from Coal
D3180 Practice for Calculating Coal and Coke Analyses from As-Determined to Different Bases
D7348 Test Methods for Loss on Ignition (LOI) of Solid Combustion Residues
D7582 Test Methods for Proximate Analysis of Coal and Coke by Macro Thermogravimetric Analysis
D8146 Guide for Evaluating Test Method Capability and Fitness for Use
E691 Practice for Conducting an Interlaboratory Study to Determine the Precision of a Test Method
2.2 ISO Standard:
ISO/IEC Guide 99:2007 International vocabulary of metrology -- Basic and general concepts and associated terms (VIM)
3. Terminology
3.1 For definitions of terms used in this test method, refer to Terminology D121.
4. Summary of Test Method
4.1 The sample to be analyzed is ashed under standard conditions and ignited to constant weight.mass. The ash is fused with a
fluxing agent followed by dissolution of the melt in dilute acid solution. Alternatively, the ash is digested in a mixture of
hydrofluoric, nitric, and hydrochloric acids. The solution is analyzed by inductively coupled plasma-atomic emission spectrometry
(ICP) for the elements. The basis of the method is the measurement of atomic emissions. Aqueous solutions of the samples are
nebulized, and a portion of the aerosol that is produced is transported to the plasma torch where excitation and emission occurs.
Characteristic line emission spectra are produced by a radio-frequency inductively coupled plasma. A grating monochromator
system is used to separate the emission lines, and the intensities of the lines are monitored by photomutilplier tube or photodiode
array detection. The photocurrents from the detector are processed and controlled by a computer system. A background correction
technique is required to compensate for variable background contribution to the determination of elements. Background must be
measured adjacent to analyte lines of samples during analysis. The position selected for the background intensity measurement,
on either or both sides of the analytical line, will be determined by the complexity of the spectrum adjacent to the analyte line.
The position used must be free of spectral interference and reflect the same change in background intensity as occurs at the analyte
wavelength measured.
5. Significance and Use
5.1 A compositional analysis of coal and coke and their associated combustion residues are often useful in assessing their quality.
Knowledge of the elemental composition of the associated residues is also useful in predicting the elemental enrichment/depletion
compositional behavior of ashes and slags in comparison to the concentration levelsmass fraction in the parent coal. Utilization
of the ash by-products and hazardous potential may also depend on the chemical composition and leachability of the inorganic
constituents of the coal ash.
5.2 The chemical composition of laboratory-prepared ash may not exactly represent the composition of mineral matter in coal or
the composition of fly ash and slag resulting from commerical-scale burning of the coal.
6. Interferences
6.1 Several types of interference effects may contribute to inaccuracies in the determination of major and minor elements. The
analyst should follow the manufacturer’s operating guide to develop and apply correction factors to compensate for the
interferences. The interferences can be classified as spectral, physical, and chemical.
6.1.1 Spectral interferences can be categorized as overlap of a spectral line from another element, unresolved overlap of molecular
band spectra, background contribution from continuous or recombination phenomena, and background contribution from stray
light from the line emission of high mass concentration elements. The second effect may require selection of an alternate
wavelength. The third and fourth effects can usually be compensated by a background correction adjacent to the analyte line. In
addition, users of simultaneous multi-element instrumentation must assume the responsibility of verifying the absence of spectral
interference from an element that could occur in a sample but for which there is no channel in the instrument array.
6.1.2 Table 1 lists the elements determined by this method and the recommended wavelengths using conventional nebulization.
Sulfur may only be determined if the sample is dissolved by the mixed acid dissolution described in 10.3.2.
Available from International Organization for Standardization (ISO), 1 rue de Varembé, Case postale 56, CH-1211, Geneva 20, Switzerland, http://www.iso.ch.
D6349 − 21
TABLE 1 Recommended Wavelengths for Elements Determined
by ICP
Element Wavelengths, nm
Aluminum 396.152, 256.80, 308.215, 309.271
Barium 455.403, 493.41, 233.53
Calcium 317.93, 315.887, 364.44, 422.67
Iron 259.940, 271.44, 238.204
Magnesium 279.553, 279.08, 285.21, 277.983
Manganese 257.610, 294.92, 293.31, 293.93
Phosphorous 178.287, 214.900
Potassium 766.491, 769.896
Silicon 212.412, 288.16, 251. 611
Sodium 588.995, 589.592
Strontium 421.55
Sulfur 182.04
Titanium 337.280, 350.50, 334.941
6.1.3 Table 2 lists some interference effects for the recommended wavelengths given in Table 1. The data in Table 2 are intended
for use only as a rudimentary guide for the indication of potential spectral interferences. For this purpose, linear relations between
mass concentration and intensity for the analytes and the interferents can be assumed. The analyst should follow the manufacturer’s
operating guide to develop and apply correction factors to compensate for the interferences.
6.1.4 Physical interferences are generally considered to be effects associated with the sample nebulization and transport processes.
Such properties as change in viscosity and surface tension can cause significant inaccuracies, especially in samples that may
contain high dissolved solids or acid concentrations, or both. The use of a peristaltic pump is recommended to lessen these
interferences. If these types of interferences are operative, they must be reduced by dilution of the sample or utilization of standard
addition techniques, or both. Another problem that can occur from high dissolved solids is salt buildup at the tip of the nebulizer.
This affects aerosol flow rate causing instrumental drift. WettingHumidifying the argon before nebulization, the use of a tip washer,
or sample dilution have been used to control this problem. Also, it has been reported that better control of the argon flow rate,
particularly nebulizer flow, improves instrument precision. This is accomplished with the use of mass flow controllers.
6.1.5 Chemical interferences are characterized by molecular compound formation, ionization effects, and solute vaporization
effects. Normally these effects are not pronounced with the ICP technique. However, if such effects are observed they can be
minimized by careful selection of operating conditions (that is,(such as, incident power, gas flows, observation position, and so
forth), height, and sample uptake rate), by buffering of the sample, matrix matching, and standard addition procedures. These types
of interferences can be highly dependent on matrix type and the specific analyte element.
7. Apparatus
7.1 Ashing Furnace, with an adequate air circulation and capable of having its temperature regulated at 500°C500 °C and
750°C.750 °C.
7.2 Fusion Furnace, with an operating temperature of 10001000 °C to 1200°C.1200 °C.
7.3 Meker-Type Burner, with inlets for fuel gas (propane or natural gas) and compressed air, capable of flame temperatures of
10001000 °C to 1200°C.1200 °C.
7.4 Platinum Dishes or Crucibles, 35-35 mL to 85-mL85 mL capacity. Graphite crucibles with 10-10 mL to 15-mL15 mL capacity
may also be used.
7.5 Stirring Hotplate and Bars, with operating temperature up to 200°C.200 °C.
7.6 Polycarbonate Bottles, 250-mL250 mL capacity with an O-ring seal and screw cap, capable of withstanding temperatures of
Methods for Chemical Analysis of Water and Wastes , (EPA-600/4-79-020), Metals-4, Method 200.7 CLP-M.
D6349 − 21
TABLE 2 Examples of Analyte Mass Concentration Equivalents (mg/L) Arising from Interference at the 100-ppm (mg/L)100 mg/L Level
NOTE 1—Dashes indicate that no interference was observed even when interferents were introduced at the following levels: Al, Ca, and Fe =
10001000 mg ppm, ⁄L, Mn = 200200 mg ppm, ⁄L, and Mg = 100 100 mg ppm.⁄L.
Interferents
Analyte Elements Wavelengths, nm Al Ca Fe Mg Mn Ti
Aluminum 308.215 - - - - - - - - - - - - 0.21 - - -
Barium 455.103 - - - - - - - - - - - - - - - - - -
Calcium 317.933 - - - - - - 0.01 0.01 0.04 0.03
Iron 259.940 - - - - - - - - - - - - 0.12 - - -
Magnesium 279.079 - - - 0.02 0.13 - - - 0.25 0.07
Manganese 257.610 0.005 - - - 0.002 0.002 - - - - - -
Silicon 288.148 - - - - - - - - - - - - - - - - - -
Sodium 588.995 - - - - - - - - - - - - - - - 0.08
100100 °C to 130°C,130 °C, the pressure that is developed during the digestion, and resistant to oxidation. Other types of bottles
or vials may be used provided they are capable of withstanding the temperatures and pressures developed duingduring the
digestion.
7.7 Inductively Coupled Plasma-Atomic Emission Spectrometer (ICP), either a sequential or simultaneous spectrometer is suitable.
Because of the differences between various makes and models of satisfactory instruments, no detailed operating instructions can
be provided. Instead, the analyst should follow the instructions provided by the manufacturer of the particular instrument.
Sensitivity, instrumental detection limit, precision, linear dynamic range, and interference effects must be investigated and
established for each individual analyte line on that particular instrument. All measurements must be within the instrument’s linear
range in which correction factors are valid. It is the responsibility of the analyst to verify that the instrument configuration and
operating conditions used satisfy the analytical requirements of this method and to maintain quality control data confirming
instrument performance and analytical results.
NOTE 2—The abbreviation ICPAES is used throughout this test method to refer to Inductively Coupled Plasma Atomic Emission Spectrometry, and it is
understood that some manufacturers will instead use the abbreviation ICPOES. In all cases, it is understood that ICPAES and ICPOES refer to the same
technique.
8. Reagents
8.1 Purity of Reagents—ReagentsReagent grade chemicals shall be used in all tests. It is intended that all reagents shall conform
to the specifications of the Committee on Analytical Reagents of the American Chemical Society in which such specifications are
available. Other grades may be used provided it is first ascertained that the reagent is of sufficiently high purity to permit its use
without lessening the accuracy of the determination.
8.2 Purity of Water—Unless otherwise indicated, references to water shall be understood to mean Type II reagent water as defined
by Specification D1193.
8.3 Standard Stock Solutions—Stock solutions of 10001000 mg ppm (mg/L) ⁄L for each element are needed for preparation of
dilute standards in the range from <0.1< 0.1 mg ⁄L to 100100 mg ppm. ⁄L. Prepare standard stock solutions from 99.999 %99.99 %
purity metals or salts. Alternatively, one can use commercially available stock solutions specifically prepared for ICP-AES
spectroscopy.
8.3.1 If multi-element stock standards are prepared, care should be taken to take into account contamination present for other
elements of interest in each single element stock solution. It is recommended that multi-element stocks be purchased from a
reputable standards vendor and include a certification of the major element mass concentrations as well as a table of contaminant
elements and mass concentrations.
8.4 Internal Standard Solution—Stock solution of 10001000 mg ppm (mg/L) ⁄L of yttrium (Y), scandium (Sc), indium (In), or
other suitable element not found in significant concentrations mass fractions in the test samples.
Reagent Chemicals, American Chemical Society Specifications, , American Chemical Society, Washington, DC. For suggestions on the testing of reagents not listed by
the American Chemical Society, see Analar Standards for Laboratory Chemicals, BDH Ltd., Poole, Dorset, U.K., and the United States Pharmacopeia and National
Formulary, U.S. Pharmacopeial Convention, Inc. (USPC), Rockville, MD.
D6349 − 21
8.5 Acids:
8.5.1 Hydrochloric Acid—Concentrated HCl. sp gr hydrochloric acid, 12 N, specific gravity 1.19.
8.5.2 Hydrofluoric Acid—Concentrated HF, sp gr hydrofluoric acid, 29 N, specific gravity 1.17.
8.5.3 Nitric Acid—Concentrated HNOnitric , sp gr acid, 16 N, specific gravity 1.42.
8.5.4 Nitric Acid (5 + 95)—Dilute 50 mL of concentrated nitric acid to 1000 mL.
8.5.5 Mixed Acid Solution, 70/30 HCl/HF—Mix seven parts concentrated hydrochloric acid and three parts concentrated
hydrofluoric acid.
8.6 Fluxing Agents— Lithium tetraborate, Li B O , or mixtures of lithium tetraborate (Li B O ) and anhydrous lithium
2 4 7 2 4 7
metaborate (LiBO ).
8.7 Boric Acids Solution—1.5 %.
8.8 Hydrogen Peroxide—30%30 %.
8.9 Wetting Agents—Approximately 0.1 g of reagent grade lithium iodide (LiI) or other suitable wetting agent may be added to
the flux to facilitate pooling of the melt and removal of the melt of cooled pellet.
8.10 Standard Solution Diluent—Use either 8.10.1 or 8.10.2.
8.10.1 WeighTransfer 4 g, determined to the nearest 0.0001 g, of fluxing agent (see 8.6) into a clean 1000-mL1000 mL beaker
containing a magnetic stirring bar. Add 500 mL of 5 + 95 nitric acid (see 8.5.4) to the beaker and place on a stirring hot plate. Heat
the mixture to just below boiling and maintain this temperature with constant stirring until the fluxing agent dissolves. This
dissolution process should take about 30 min or less (see Note 13). Quantitatively transfer the warm solution to a
1000-mL1000 mL volumetric flask. After the solution cools to room temperature, dilute to 1000 mL with reagent grade water.
8.10.2 WeighTransfer 4 g, determined to the nearest 0.0001 g, of fluxing agent (see 8.6) into a platinum dish (or crucible). Heat
to 1000°C1000 °C to form a liquid and cool. Carefully rinse the bottom and outside of the platinum dish to remove possible
contamination. Place the cooled platinum dish containing the flux and a magnetic stirring bar into a clean 1000-mL1000 mL
beaker. Add 500 mL of 5 + 95 nitric acid (see 8.5.4) to the beaker and place immediately on the stirring hotplate. Heat the mixture
to just below the boiling temperature and maintain this temperature with constant stirring until the melt dissolves. This dissolution
process should take about 30 min 30 min (see Note 13). After dissolution, remove the platinum dish after rinsing with reagent water
and collecting the washings in the acid solution. Quantitatively transfer the warm solution to a 1000-mL1000 mL volumetric flask.
After the solution cools to room temperature, dilute to 1000 mL 1000 mL with reagent grade water.
NOTE 3—This time and temperature are sufficient to dissolve the melt completely. If stirring is not maintained constantly, some of the material may not
dissolve, and the final solution must be filtered before use.
8.11 Blank Solutions—All of the test methods in this standard require two types of blank solutions. A calibration blank that is used
to establish the analytical calibration curve and a method blank which is used to evaluate possible contamination and assess
spectral background. The calibration blank is also used initially and periodically to verify the baseline of the calibration has not
changed significantly.
8.11.1 Calibration Blank—The same solution as the Standard Solution Diluent.
8.11.2 Method Blank—The method blank shall be processed through the same digestion procedure as the samples and consist of
all the reagents in the same volumes as used in preparing the samples.
8.12 Initial calibration verification standard(s):Calibration Verification Standard(s):
D6349 − 21
8.12.1 Where possible the initial calibration verification standard(s) shall be from alternate producers or different lot numbers from
the calibration standard(s).
8.12.2 Where possible the initial calibration verification standard(s) shall be traceable to a primary standard such as a NIST
CRM.National Institute of Technology Standard Reference Material (NIST SRM).
8.13 Periodic calibration verification standard(s)—Calibration Verification Standard(s)—The source of these materials can be the
same as the calibration materials.
8.14 Primary Control Sample—A material that is processed following the same procedure as an analytical sample and is a
measurement standard whose quantity value and measurement uncertainty are established without relation to another measurement
standard for a quantity of the same kind (see ISO/IEC Guide 99:2007 International Vocabulary of Basic and General Terms in
Metrology).
8.15 Secondary Control Sample—A material that is processed following the same procedure as an analytical sample and is a
measurement standard whose quantity value and measurement uncertainty are assigned through calibration against, or comparison
with, a primary measurement standard for a quantity of the same kind (see ISO/IEC Guide 99:2007 International Vocabulary of
Basic and General Terms in Metrology).
9. Sample Preparation
9.1 Coal and Coke—Prepare the analysis sample in accordance with Practice D2013 for coal or Practice D346 for coke by
pulverizing the material to pass a 250 μm (No. 60) U.S.A. standard sieve.
9.1.1 Analyze separate test portions for moisture and ash contents in accordance with Test Methods D3173, D3174, or D7582 so
that calculation to other bases can be made.
9.2 Laboratory Ashing of Coal and Coke Analysis Sample—Prepare the ash from a thoroughly mixed analysis sample of coal or
coke (see 9.1). Spread the coal and coke in a layer not over 6 mm in depth in a porcelain, quartz, or fused silica roasting dish. Place
the dish in a cold muffle furnace and heat gradually so that the temperature reaches 500500 °C 6 10°C10 °C at the end of 1 h.
Continue the gradual heating until the temperature rises from 500500 °C 6 10°C10 °C to 750750 °C 6 15°C15 °C at the end of
1 h. Maintain the 750°C750 °C temperature until the test specimen reaches a constant mass or for an additional two hours. 2 h.
Allow the dish to cool, transfer to an agate mortar, and grind to pass a 75 μm (No. 200) U.S.A. standard sieve. Reignite the ash
at 750°C750 °C for 1 h, cool rapidly, and weigh allow to cool to room temperature, and determine the mass of portions for analysis.
9.3 Solid Combustion Residue—Dry a representative portion of the solid residue to constant mass at 107107 °C 6 3°C.3 °C.
Determine the moisture loss during this drying step if it is desirable to calculate results to an as-received basis. Crush the dried
portion of the sample to pass a 75 μm (No. 200) U.S.A standard sieve. Use a mill that minimizes metal contamination.
9.4 Ashing Solid Combustion Residue—Spread an appropriate amount of the prepared sample in a layer not over 2 mm in a
porcelain, quartz, or fused silica roasting dish. Place the dish in a cold muffle furnace and heat gradually so that the temperature
reaches 500500 °C 6 10°C10 °C at the end of 1 h. Continue the gradual heating until the temperature rises from 500500 °C 6
10°C10 °C to 750750 °C 6 15°C15 °C at the end of 1 h. Maintain the 750°C750 °C temperature until the combustion residue
reaches a constant mass or for an additional two hours. 2 h. Cool the test specimen, grind to pass a 75 μm (No. 200) U.S.A standard
sieve, and reignite at 750°C750 °C for 1 h.
9.5 If previously-ignited samples are stored and the absorption of moisture, or CO , or both, is in question, reignite the ash at
750°C750 °C before use. Alternatively, determine loss on ignition using Test Method D7348 on a separate sample weighed out
whose mass is determined at the same time as the test portion and make the necessary corrections. Thoroughly mix each sample
before weighing.
10. Procedure
10.1 The solutions and proportions described below are the typical ash samples as represented by American coals. Therefore,
stronger or weaker dilutions may be required to establish suitable mass concentrations for those elements of varying percents
D6349 − 21
outside the range of the typical sample. Analysts must determine the sensitivity and linear range of calibration of their own
equipment and choose mass concentration ranges for standards compatible with the samples and instrument specific to their own
work.
10.2 To minimize the potential of contamination, platinum ware must be prepared by boiling in dilute HNO (5 + 95) and rinsing
thoroughly with reagent-grade water. After this initial cleaning, the platinum ware must be handled with clean tongs and protected
from contamination from table tops, tabletops, and so forth. All glassware used in analyses must be equally clean and protected.
10.3 Ash Dissolution—Two methods of dissolving the ash samples are offered for this test method. method: fusion and mixed acid.
The analyst may choose the method most appropriate for their laboratory and instrumentation. Laboratories using the fusion
method (see 10.3.1) for dissolving the ash should be aware that a considerable amount of sulfur may be lost from the ash during
the fusion process. A blank test solution containing the same mass concentration of reagents used for the ash samples shall be
prepared and analyzed with the ash sample solutions.
10.3.1 Sample Fusion and Dissolution—Weigh 0.1g (to Transfer 0.1 g (determined to the nearest 0.1mg)0.1 mg) of the ash sample
as prepared in 9.5 or 9.4 into a platinum dish (or crucible) (see Note 24). Weigh 0.4g (to Transfer 0.4 g (determined to nearest 0.5
mg) of the fluxing agent and add to the ash sample. Mix the ash and fluxing agent thoroughly and heat to melting at 10001000 °C
to 1200°C1200 °C with stirring, according to 10.3.1.1 or 10.3.1.2, until a clear melt is obtained.
10.3.1.1 If a muffle furnace is used for heating, place the platinum dish in a clean silica or refractory tray and place in a muffle
furnace preheated to 1000°C;1000 °C; 7 min at this temperature is sufficient to fuse most mixtures completely, but heating should
be continued until a clear pellet is obtained. Use platinum-tipped tongs to swirl the melt gently to dissolve the ash. Remove the
tray with the dish and cool to room temperature. Carefully rinse the bottom and outside of the platinum dish to remove possible
contamination; then place is in a clean 250-250 mL or 400-mL400 mL beaker. Place a clean TFE-fluorocarbon coated magnetic
stirring bar in the platinum dish and add 50 mL 50 mL of 5(5 + 95 HNO95) nitric acid (see 8.5.4) to the melt in the platinum dish.
Immediately place the beaker with the dish on the stirring hotplate. Stir and heat the solution to just below boiling and maintain
this near boiling condition until the melt is dissolved or for not more than 30 min 30 min. Constantly stir and heat the solution,
and repeat the analysis if precipitate is formed (see Note 35). Remove the platinum dish from the beaker, rinse the dish with small
amounts of reagent water, and quantitatively transfer the solution to a 100-mL100 mL volumetric flask. Add 1 mL of internal
standard to the flask and dilute to the 100-mL100 mL mark with water. This solution is 10001000 mg ppm ⁄L with respect to the
total sample and contains 44 g g/L ⁄L of fluxing agent. Prepare a method blank using the above procedure.
NOTE 4—Graphite crucibles may be used instead of platinum for the fusion. The graphite crucibles are not to be immersed in the digestion solution. Pour
the red-hot melt directly from the crucible into the acid solution and proceed with stirring and heating as written above.
NOTE 5—If the stirring is not constantly maintained, some of the constituents may precipitate, primarily silicic acid, as a result of heating in the highly
acidic solution. The analysis must then be repeated.
10.3.1.2 If a flame is used for heating, rotate the platinum dish in the flame until a clear melt is obtained. If automated fusion
equipment is being used, follow the manufacturer’s programmed steps. If the crucible is inserted manually into the flame using
platinum-tipped tongs, stir by swirling for at least 5 min. When a clear melt is obtained, either pour the hot melt into 50 mL 50 mL
of 5(5 + 9595) nitric acid (see 8.5.4) in a clean 250-250 mg ⁄L or 400-mL400 mL beaker containing a Teflon-coatedTFE-
fluorocarbon magnetic stirring bar or cool the crucible and transfer the solid pellet to this solution. (It is the analyst’s responsibility
to ensure that the entire sample is transferred to the nitric acid solution). If it cannot be completely transferred, the sample should
be discarded and re-prepared. Immediately place the beaker on a stirring hot plate. Stir and heat the solution to just below boiling
and maintain the near boiling condition until the pellet is dissolved or for not more than 30 min 30 min. Constantly stir and heat
the solution, and repeat the analysis if precipitate is formed (see Note 35). Transfer the solution quantitatively to a 100-mL100 mL
volumetric flask. Add 1 mL 1 mL of internal standard to the flask and dilute to the 100-mL100 mL mark with water. This solution
is 1000 ppmmg/L with respect to the total ash sample and contains 4 g/L of fluxing agent. Prepare a method blank using the above
procedure.
10.3.2 Mixed Acid Dissolution—Weigh 0.1g (to Transfer 0.1 g (determined to the nearest 0.1 mg) of the ash sample as prepared
in 9.5 or 9.4 into a 250-mL250 mL polycarbonate bottle with an O-ring seal and screw cap 9see cap. Note 4. The bottle should
be capable of withstanding a temperature up to 130°C,130 °C, the pressure developed during digestion, and resistant to oxidation.
(Warning—With repeated use the polycarbonate bottles will become brittle and develop cracks. They should be inspected before
each use. A convenient way to do this is to hold them up to a light source. If any evidence of cracks is noted, the bottle should
be discarded.)
D6349 − 21
NOTE 4— Some combustions residues may contain sulfite sulfur. If sulfite is known to be present or is suspected, add 1-mL of 30% H O to the digestion
2 2
bottle before proceeding to 10.3.2.1. The peroxide will oxidize sulfite species to sulfate which is quantitatively retained in the digestion process. If
peroxide is added, make the appropriate adjustment to the final sample volume used in the calculation of results in Section 12.
10.3.2.1 If sulfite is known to be present or is suspected, add 1 mL of 30 % peroxide to the digestion bottle (see Note 4) before
proceeding to 10.3.2.2. If 30 % peroxide is added, make the appropriate adjustment to the final sample volume (for example,
101 mL versus 100 mL) used in the calculation of results in Section 12.
NOTE 6—The peroxide will oxidize sulfite species to sulfate which is quantitatively retained in the digestion process.
10.3.2.2 Add 5.0 mL of the 70/30 HCl/HF mixed acid solution (8.5.5) and 2.0 mL of concentrated HNO to the sample and tighten
the screw cap (see Note 57). Heat the bottle at 100100 °C to 130°C130 °C in a boiling water bath, on a steam bath, or in an oven
at 100 °C to 130 °C for at least 2 h. Remove the bottle from the heat source, and add 93.01.0 mL of internal standard and 92.0
mL of 1.5 % boric acid (H BO ) solution. Tighten the screw cap. Return the bottle to the heat source and continue heating for 1
3 3
h. Cool the solution to room temperature before analysis. If the samples are not analyzed immediately, they may be stored in their
original digestion bottles or transferred to polyethylene bottles. Prepare a method blank using the above procedure.
NOTE 7—The 70/30 HCl/HF mixed acid solution (see 8.5.5) can be prepared and stored until use, whereas an aqua regia mixture (HCl and HNO ) is not
stable. Using the mixed acid solution and concentrated HNO is equivalent to using aqua regia and HF.
10.3.3 Prepare calibration standards using appropriate values of standard stock solutions (see 8.3). Add 1-mL1 mL internal
standard solution (see 8.4) per 100-mL100 mL volume used. Dilute to the mark with the proper diluents.matrix-matched diluents
(see 11.2.2.2).
10.3.4 If instrumentation is capable of continuous addition of internal standard to both samples and standards, then samples and
standards can be prepared without the internal standard so long as volumes are correctly accounted for in subsequent calculations
of mass concentration.
11. Instrument Operation
11.1 Consult the manufacturer’s instructions for operation of the ICP spectrometer. The present method assumes that good
operating procedures are followed. Design differences among instruments and different selected analytical wavelengths for
individual spectrometers make it impractical to list detailed conditions.
11.2 To ensure the validity of the data obtained from an ICP analysis, the following QC elements shall be considered the minimum
for each analyte wavelength.
11.2.1 Initial and periodic instrument performance verification (also to be performed after major maintenance):
11.2.1.1 All manufacturer specified spectral alignment practices (such as Mercury lamp alignment) alignment and viewing
alignment procedures) shall be followed.
11.2.1.2 The alignment reference peak intensity shall be monitored following the manufacturer recommendations. A Mangane-
semanganese solution is often used for this purpose.
11.2.1.3 The minimum detectable limit shall be verified every 6 months for analytes previously determined to be within ten times
the minimum detectable limit. The minimum detectable limit must be less than or equal to the reporting limit.
11.2.1.4 Select peak wavelengths to minimize/eliminate spectral interferences.
11.2.1.5 Inter-element interference corrections (spectral interferences) and and other types of corrections for spectral interferences
(such as schematical models used for correction of spectral overlaps) and background point corrections shall be verified every 3
months according to manufacturer specifications.
11.2.2 Calibration:
D6349 − 21
TABLE 3 Examples of Calibration Standard Mass Concentrations
to Prepare Based on Estimated Ash Sample Mass
Concentrations
Analyte Calcium Silicon
Estimated sample mass Calcium 10 mg ⁄L to 180 mg ⁄L to 230 mg ⁄L
concentration 20 mg ⁄L in solution in solution
Recommended middle 15 mg/L 200 mg/L
standard mass
concentration
Recommended high 30 mg/L 400 mg/L
standard mass
concentration
Recommended low 1.5 mg/L 20 mg/L
standard mass
concentration
11.2.2.1 All analysis results must fall within the mass concentration range of the calibration standards. If a sample result occurs
above the high calibration standard, dilute the sample and reanalyze for that element.
11.2.2.2 All calibration solutions shall be matrix matched (in relation to the dissolution background such as LiB O and acids) acid
4 7
type and concentrations) to the ash sample solutions. See Note 8
NOTE 8—In order to compensate for physical interferences such as viscosity differences between calibration standards and samples that may affect
intensities of the elements being analyzed, the use of an internal standard is permitted. The internal standard should be added to all calibration blanks,
standards, QC samples, and samples at the same mass concentration and should be an element not present in any sufficient quantity in the samples.
Elements such as Sc, In, and Y are routinely used. The internal standard can either be spiked into each solution or alternatively added online through the
use of a mixing tee just prior to the sample solution entering the nebulizer.
11.2.2.3 The calibration shall include a minimum of a calibration blank and three calibration standard mass concentrations,
assuming a linear calibration. See examples in Table 3. The recommended relative mass concentrations for the calibration standards
are:
(1) The middle standard should be near the mid-point of the expected sample mass concentration range.
(2) The high standard should be approximately two times the middle standard standard.
(3) The low standard should be approximately one-tenth ( ⁄10) of the middle standard.
(4) The linear correlation of the calibration regression shall be 0.995 or greater.
Analyte Calcium Silicon
Estimated sample Calcium 10 mg/L to 180 mg/L to
concentration 20 mg/L in solution 230 mg/L in solution
Recommended middle 15 mg/L 200 mg/L
standard concentration
Recommended high 30 mg/L 400 mg/L
standard concentration
Recommended low 1.5 mg/L 20 mg/L
standard concentration
11.2.3 Initial calibration verification:Calibration Verification:
11.2.3.1 A successful calibration shall be verified with an initial calibration verification standard(s) and the calibration blank prior
to the analysis of any samples.
11.2.3.2 The initial calibration verification recovery shall be within 5%5 % of the known value. See 8.12 and 8.13.
11.2.3.3 The initial calibration blank reported mass concentration shall be below the reporting limit.
11.2.4 Periodic calibration verification:Calibration Verification:
th
11.2.4.1 The calibration shall be verified after every 10th10 analysis and at the end of the batch or shift using a periodic
calibration verification standard(s) and a calibration blank.
11.2.4.2 The periodic calibration verification recovery shall be within 10%10 % of the known value. See 8.12 and 8.13.
D6349 − 21
11.2.4.3 The periodic calibration blank reported mass concentration shall be below the reporting limit.
11.2.5 If a calibration verification fails to meet the criteria, it shall be rerunre-analyzed once. If it still fails, the calibration is
suspect and any samples analyzed after the last acceptable calibration verification shall be re-analyzed. re-analyzed after the
instrument has been recalibrated.
11.2.6 Preparation batchBatch Quality Control Checks:
11.2.6.1 Various checks are necessary to ensure that the dissolution process applied to the samples provides accurate recovery
without contamination.
11.2.6.2 All preparation batch quality control shall be performed once for each batch or for every 40 samples, whichever is more
frequent.
11.2.6.3 Method blank—Blank—Absolute results for this blank shall be less than the reporting limit.
11.2.6.4 Preparation duplicates—Duplicates—A sample prepared in duplicate following the procedure in section 10 of this
standard. If the duplicates fail to meet the repeatability specifications of this test method, reanalyze the sample solution and the
duplicate solution once. If these results still fail to meet the repeatability specifications consider the preparation batch in question
and investigate the problem.
11.2.6.5 Secondary Control sample—Sample—A secondary measurement standard shall be processed following the procedure in
section 10Section 10 of this standard. test method. Results for the control sample shall be within the ASTM Reproducibility limits
and within the laboratory’s process control limits (as defined in ASTM Manual 7 or other appropriate process control limit
definition).
11.2.6.6 Primary Control sample—Sample—A primary measurement standard shall be processed through the entire sample
digestion scheme. This sample shall be performed a minimum of once per quarter. Results for the primary control sample shall
be within the ASTM Reproducibility limit and within the laboratory’s process control limit (as defined in ASTM Manual 7 or other
appropriate process control limit definition).
11.2.7 Secondary QC verifications—Verifications—Post analysis verifications include verification of the sum of the oxides as a
weight percentpercent mass fractions of the sample. The undetermined content shall not exceed 5%5 % when all major and minor
analytes and SO3SO are included. If the undetermined value exceeds 5%,5 %, the analysis shall be considered suspect, and
verification steps shall be taken when the cause for a high undetermined percent is not already known.
12. Calculation or Interpretation of Results
12.1 Calculate the percentage (by weight) mass fraction of each element in the ash using the following equation:
% E 5 ~C 3V!/W 3D 3100 (1)
100CVD
E 5 (1)
W
where:
E = element analyzed,
C = concentration in mg/L (ppm or g/g) of M in the analyzed solution,
V = volume (in litres) of sample solution prepared in Section 10,
W = weight of sample in milligrams, and
D = dilution factor; = final volume of analyzed solution divided by the amount of the prepared solution (see Section 10) used
for the dilution.
E = mass fraction of element analyzed, %,
100 = conversion factor from dimensionless mass fraction to percent, %,
C = mass concentration of the element in the analyzed solution, mg/L,
Manual of Presentation of Data and Control Chart Analysis, ASTM MNL7A, ASTM International, 2002.
D6349 − 21
V = volume of sample solution prepared in Section 10, L,
W = mass of ash sample, mg, and
D = dilution factor; final volume of analyzed solution divided by the volume of the prepared solution (see 11.2.2.1) used for
the dilution.
12.2 Use Practice D3180 to calculate results to other bases.
12.3 Convert mass fractions in the ash to the dry sample basis for reporting as follows:
C 5 A 3B/100 % (2)
AB
E 5 (2)
where:
C = percent of elemental oxide (dry basis) in the sample,
A = percent of elemental oxide determined in the ash, and
B = % (dry basis) ash in the sample.
E = mass fraction of elemental oxide in the sample, % dry basis,
A = mass fraction of elemental oxide determined in the ash sample, %,
B = mass fraction ash in the sample, % dry basis, and,
100 = conversion factor from dimensionless mass fraction to percent, %.
13. Precision and Bias
13.1 Precision—The precision of this test method for the determination of major and minor elements in ash from coal, coke, and
solid combustion residues are shown in Table 34. The precision characterized by the repeatability (S , r) and reproducibility (S ,
r R
R) is described in Table A1.1 in the Annex A1.
NOTE 9—The precision and bias study for SO was performed only by the mixed acid dissolution (10.3.2) and does not include data from fusion and
dissolution (10.3.1). This study did not include combustion residues derived from coke.
13.1.1 Repeatability Limit (r)—The value below which the absolute difference between two test results of separate and consecutive
test determinations, carried out on the same sample in the same laboratory by the same operator using the same apparatus on
samples taken from a single quantity of homogeneous material, may be expected to occur with a probability of approximately
95 %.
13.1.2 Reproducibility Limit (R)—The value below which the absolute difference between two test results, carried out in different
laboratories using samples taken at random from a single quantity of material that is as nearly homogeneous as possible, may be
expected to occur with a probability of approximately 95 %.
13.2 Bias—A standard reference material (1633b-coal fly ash) from the National Institute for Standards and Technology (NIST)
reference material (SRM 1633b Constituent Elements in Coal Fly Ash) from NIST was included in the ICP interlaboratory study
to ascertain possible bias between reference material values and those determined by the new method. A comparison of the NIST
values and those obtained in the interlaboratory study are given in Table 45. The results show a very small (positive) bias for the
reported values for iron.
13.3 An interlaboratory study, designed consistent with Practice E691, was conducted in 1997, and twelve labs participated.
13.4 A second interlaboratory study, designed consistent with Practice E691, was conducted in 2003. The purpose of the
interlaboratory study was to include sulfur in the suite of elements analyzed by ICP-AES. The details of the study and supporting
data are given in a Research Report filed at ASTM headquarters.
14. Keywords
14.1 coal; coal ash; inductively coupled plasma-atomic emission spectrometry; major and minor elements
D6349 − 21
TABLE 3 Concentration Ranges and Limits for Repeatability and Reproducibility for Major and Minor Elemental Oxides in Ash from
Coal, Coke, and Solid Combustion Residues
NOTE 1—The precision and bias study for SO3 was performed only by the mixed acid dis
...







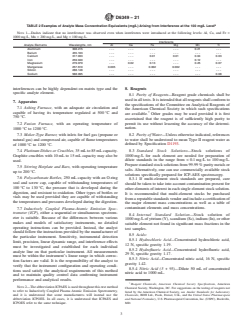


Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.
Loading comments...