ASTM D3558-94(1998)
(Test Method)Standard Test Methods for Cobalt in Water
Standard Test Methods for Cobalt in Water
SCOPE
1.1 These test methods cover the determination of dissolved and total recoverable cobalt in water and wastewater by atomic absorption spectrophotometry. Three test methods are included as follows: Concentration Range Sections Test Method A-Atomic Absorption, Direct 0.1 to 10 mg/L 7 to 15 Test Method B-Atomic Absorption, Chelation-Extraction 10 to 1000 [mu]g/L 16 to 24 Test Method C-Atomic Absorption, Graphite Furnace 5 to 100 [mu]g/L 25 to 33
1.2 Test Method A has been used successfully with reagent water, potable water, river water, and wastewater. Test Method B has been used successfully with reagent water, potable water, river water, sea water and brine. Test Method C was successfully evaluated in reagent water, artificial seawater, river water, tap water, and a synthetic brine. It is the analyst's responsibility to ensure the validity of these test methods for other matrices.
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use. For specific hazard statements, see Notes 3, 5, 8, and 13.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superceded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
Designation: D 3558 – 94 (Reapproved 1998)
Standard Test Methods for
Cobalt in Water
This standard is issued under the fixed designation D 3558; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
This standard has been approved for use by agencies of the Department of Defense.
1. Scope D 1691 Test Methods for Zinc in Water
D 1886 Test Methods for Nickel in Water
1.1 These test methods cover the determination of dissolved
D 2777 Practice for Determination of Precision and Bias of
and total recoverable cobalt in water and wastewater by
Applicable Methods of Committee D–19 on Water
atomic absorption spectrophotometry. Three test methods are
D 3370 Practices for Sampling Water from Closed Con-
included as follows:
duits
Concentration Range Sections
D 3557 Test Methods for Cadmium in Water
Test Method A—Atomic Absorption,
Direct 0.1 to 10 mg/L 7 to 15
D 3559 Test Methods for Lead in Water
Test Method B—Atomic Absorption,
D 3919 Practice for Measuring Trace Elements in Water by
Chelation-Extraction 10 to 1000 μg/L 16 to 24
Graphite Furnace Atomic Absorption Spectrophotometry
Test Method C—Atomic Absorption,
Graphite Furnace 5 to 100 μg/L 25 to 33
D 4841 Practice for Estimation of Holding Time for Water
Samples Containing Organic and Inorganic Constituents
1.2 Test Method A has been used successfully with reagent
water, potable water, river water, and wastewater. Test Method
3. Terminology
B has been used successfully with reagent water, potable water,
3.1 Definitions:
river water, sea water and brine. Test Method C was success-
3.1.1 For definitions of terms used in these test methods,
fully evaluated in reagent water, artificial seawater, river water,
refer to Terminology D 1129.
tap water, and a synthetic brine. It is the analyst’s responsibility
3.2 Definitions of Terms Specific to This Standard:
to ensure the validity of these test methods for other matrices.
3.2.1 total recoverable cobalt—an arbitrary analytical term
1.3 This standard does not purport to address all of the
relating to the recoverable forms of cobalt that are determin-
safety concerns, if any, associated with its use. It is the
able by the digestion method which is included in the proce-
responsibility of the user of this standard to establish appro-
dure.
priate safety and health practices and determine the applica-
bility of regulatory limitations prior to use. For specific hazard
4. Significance and Use
statements, see Note 3, Note 5, Note 8, and Note 13.
4.1 Most waters rarely contain more than trace concentra-
2. Referenced Documents tions of cobalt from natural sources. Although trace amounts of
cobalt seem to be essential to the nutrition of some animals,
2.1 ASTM Standards:
large amounts have pronounced toxic effects on both plant and
D 858 Test Methods for Manganese in Water
animal life.
D 1066 Practice for Sampling Steam
D 1068 Test Methods for Iron in Water
5. Purity of Reagents
D 1129 Terminology Relating to Water
5.1 Reagent grade chemicals shall be used in all tests.
D 1193 Specification for Reagent Water
3 Unless otherwise indicated, it is intended that all reagents shall
D 1687 Test Methods for Total Chromium in Water
conform to the specifications of the Committee on Analytical
D 1688 Test Methods for Copper in Water
Reagents of the American Chemical Society where such
These test methods are under the jurisdiction of ASTM Committee D-19 on
Waterand are the direct responsibility of Subcommittee D19.05 on Inorganic
Constituents in Water.
Current edition approved Sept. 15, 1994. Published November 1994. Originally
published as D 3558 – 77. Last previous edition D 3558 – 90.
Platte, J. A., and March, V. M., “A New Tool for the Water Chemist,” Industrial
Water Engineering, May 1965.
Annual Book of ASTM Standards, Vol 11.01.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
NOTICE: This standard has either been superceded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
D 3558 – 94 (1998)
specifications are available. Other grades may be used, 9.2 Other metals usually do not interfere in the determina-
provided it is first ascertained that the reagent is of sufficiently tion of cobalt by increasing or decreasing the amount of
high purity to permit its use without lessening the accuracy of absorbed radiation. The most common interference is caused
the determination. by a chemical reaction in the flame that prevents conversion of
5.2 Unless otherwise indicated, reference to water shall be the cobalt to the atomic state.
understood to mean reagent water conforming to Specification 9.3 Sodium, potassium, and sulfate, magnesium (4500 mg/
D 1193, Type I. Other reagent water types may be used, L), iron (4000 mg/L), and nickel, lead, copper, zinc, cadmium,
provided it is first ascertained that the water is of sufficiently and chromium (10 mg/L each) do not interfere.
high purity to permit its use without lessening the bias and 9.4 Background correction or a chelation-extraction proce-
precision of the determination. Type II water specified at the dure (see Test Method B) may be necessary to determine low
time of the round-robin testing of this test method. levels of cobalt in some water.
NOTE 1—Instrument manufacturers’ instructions for use of the specific
6. Sampling
correction technique should be followed.
6.1 Collect the samples in accordance with Practices
10. Apparatus
D 3370 and Practice D 1066, as applicable.
6.2 Preserve samples with HNO (sp gr 1.42) to a pH of 2 or
10.1 Atomic Absorption Spectrophotometer, for use at 240.7
less immediately at the time of collection, normally about 2
nm.
mL/L. If only dissolved cobalt is to be determined, filter the
NOTE 2—The manufacturer’s instructions should be followed for all
sample through a 0.45-μm membrane filter before acidification.
instrumental parameters. A wavelength other than 240.7 nm may be used
The holding time for samples may be calculated in accordance
if it has been determined to be equally suitable.
with Practice D 4841.
10.2 Cobalt Hollow-Cathode Lamps—Multielement
hollow-cathode lamps.
TEST METHOD A—ATOMIC ABSORPTION, DIRECT
10.3 Pressure-Reducing Valves—The supplies of fuel and
oxidant shall be maintained at pressures somewhat higher than
7. Scope
the controlled operating pressure of the instrument by suitable
7.1 This test method covers the determination of dissolved
valves.
and total recoverable cobalt in most waters and waste waters.
It is the user’s responsibility to ensure the validity of this test
11. Reagents and Materials
method in a particular matrix.
11.1 Cobalt Solution, Stock (1 mL = 1.0 mg Co)—Dissolve
7.2 This test method is applicable in the range from 0.1 to
4.0372 g of cobaltous chloride (CoCl ·6H O) in reagent water
2 2
10 mg/L of cobalt. The range may be extended to concentra-
and dilute to 1 L.
tions greater than 10 mg/L by dilution of the sample.
11.2 Cobalt Solution, Standard (1 mL = 0.1 mg Co)—
Dissolve 100.0 mL of the stock cobalt solution to 1 L with
8. Summary of Test Method
water.
8.1 Cobalt is determined by atomic absorption spectropho-
11.3 Hydrochloric Acid (sp gr 1.19)—Concentrated hydro-
tometry. Dissolved cobalt is determined by aspirating a portion
chloric acid (HCl).
of the filtered sample directly with no pretreatment. Total
NOTE 3—If a high reagent blank is obtained, distill the HCl or use
recoverable cobalt is determined by aspirating the sample
spectrograde acid. Caution—When HCl is distilled, an azeotrophic
following hydrochloric-nitric acid digestion and filtration. The
mixture is obtained (approximately 6 N HCl is formed). Therefore,
same digestion procedure may be used to determine total
whenever concentrated HCl is specified for the preparation of a reagent or
recoverable nickel (Test Methods D 1886), chromium (Test
in the procedure, use double the volume specified if distilled acid is used.
Methods D 1687), cadmium (Test Methods D 3557), copper
11.4 Nitric Acid (sp gr 1.42)—Concentrated nitric acid
(Test Methods D 1688), iron (Test Methods D 1068), lead (Test
(HNO ).
Methods D 3559), manganese (Test Methods D 858), zinc
NOTE 4—If a high reagent blank is obtained, distill the HNO or use a
(Test Methods D 1691). 3
spectrograde acid.
9. Interferences
11.5 Nitric Acid (1 + 499)—Add 1 volume of HNO (sp gr
1.42) to 499 volumes of water.
9.1 Nitrate reportedly interferes at 1 mg/L by suppressing
11.6 Oxidant:
the absorption of the cobalt. This interference can be elimi-
11.6.1 Air, which has been passed through a suitable filter to
nated by adding 18 000 mg/L of ammonium chloride to
remove oil, water, and other foreign substances is the usual
blanks, standards, and samples.
oxidant.
11.7 Fuel:
11.7.1 Acetylene—Standard, commercially available acety-
Reagent Chemicals, American Chemical Society Specifications, American
lene is the usual fuel. Acetone, always present in acetylene
Chemical Society, Washington, DC. For suggestions on the testing of reagents not
cylinders can affect analytical results. The cylinder should be
listed by the American Chemical Society, see Analar Standards for Laboratory
Chemicals, BDH Ltd., Poole, Dorset, U.K., and the United States Pharmacopeia
replaced at 50 psig (345 kPa).
and National Formulary, U.S. Pharmaceutical Convention, Inc. (USPC), Rockville,
MD. NOTE 5—Warning: “Purified” grade acetylene containing a special
NOTICE: This standard has either been superceded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
D 3558 – 94 (1998)
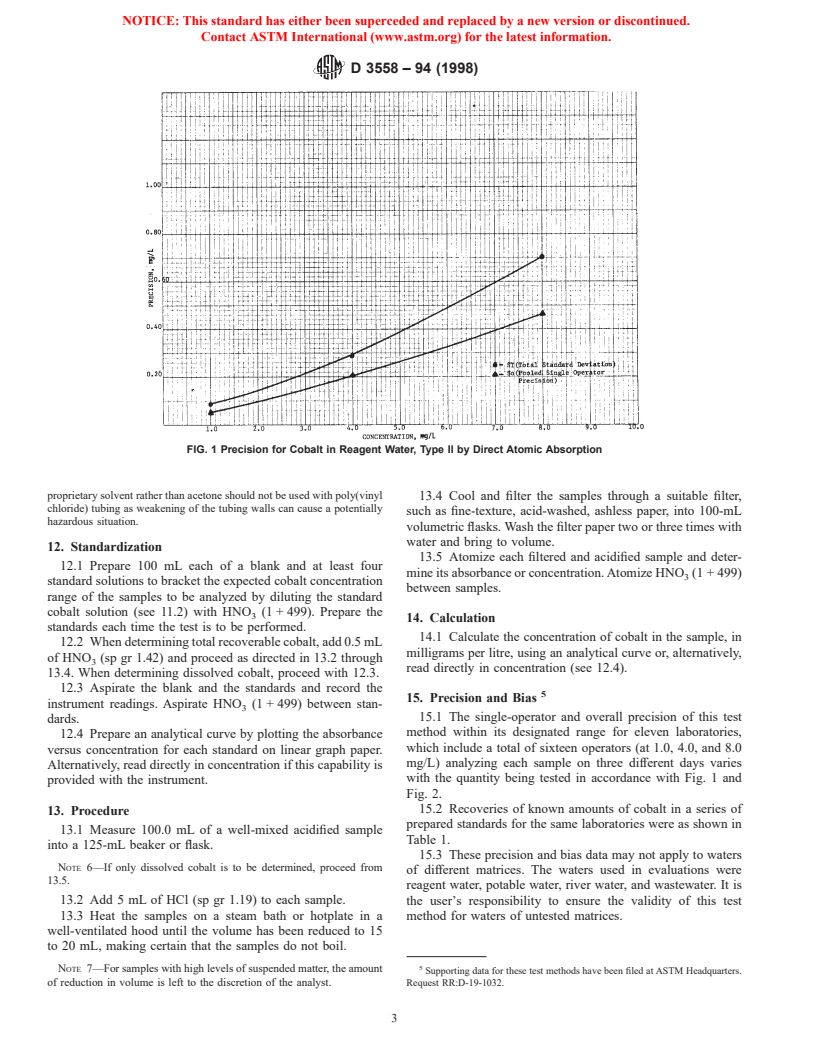
FIG. 1 Precision for Cobalt in Reagent Water, Type II by Direct Atomic Absorption
proprietary solvent rather than acetone should not be used with poly(vinyl
13.4 Cool and filter the samples through a suitable filter,
chloride) tubing as weakening of the tubing walls can cause a potentially
such as fine-texture, acid-washed, ashless paper, into 100-mL
hazardous situation.
volumetric flasks. Wash the filter paper two or three times with
water and bring to volume.
12. Standardization
13.5 Atomize each filtered and acidified sample and deter-
12.1 Prepare 100 mL each of a blank and at least four
mine its absorbance or concentration. Atomize HNO (1 + 499)
standard solutions to bracket the expected cobalt concentration
between samples.
range of the samples to be analyzed by diluting the standard
cobalt solution (see 11.2) with HNO (1 + 499). Prepare the
14. Calculation
standards each time the test is to be performed.
14.1 Calculate the concentration of cobalt in the sample, in
12.2 When determining total recoverable cobalt, add 0.5 mL
milligrams per litre, using an analytical curve or, alternatively,
of HNO (sp gr 1.42) and proceed as directed in 13.2 through
read directly in concentration (see 12.4).
13.4. When determining dissolved cobalt, proceed with 12.3.
12.3 Aspirate the blank and the standards and record the
15. Precision and Bias
instrument readings. Aspirate HNO (1 + 499) between stan-
15.1 The single-operator and overall precision of this test
dards.
method within its designated range for eleven laboratories,
12.4 Prepare an analytical curve by plotting the absorbance
which include a total of sixteen operators (at 1.0, 4.0, and 8.0
versus concentration for each standard on linear graph paper.
mg/L) analyzing each sample on three different days varies
Alternatively, read directly in concentration if this capability is
with the quantity being tested in accordance with Fig. 1 and
provided with the instrument.
Fig. 2.
15.2 Recoveries of known amounts of cobalt in a series of
13. Procedure
prepared standards for the same laboratories were as shown in
13.1 Measure 100.0 mL of a well-mixed acidified sample
Table 1.
into a 125-mL beaker or flask.
15.3 These precision and bias data may not apply to waters
NOTE 6—If only dissolved cobalt is to be determined, proceed from
of different matrices. The waters used in evaluations were
13.5.
reagent water, potable water, river water, and wastewater. It is
13.2 Add 5 mL of HCl (sp gr 1.19) to each sample. the user’s responsibility to ensure the validity of this test
13.3 Heat the samples on a steam bath or hotplate in a
method for waters of untested matrices.
well-ventilated hood until the volume has been reduced to 15
to 20 mL, making certain that the samples do not boil.
NOTE 7—For samples with high levels of suspended matter, the amount
Supporting data for these test methods have been filed at ASTM Headquarters.
of reduction in volume is left to the discretion of the analyst. Request RR:D-19-1032.
NOTICE: This standard has either been superceded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
D 3558 – 94 (1998)
FIG. 2 Precision for Cobalt in Water of Choice by Direct Atomic Absorption
TABLE 1 Recoveries of Known Amounts of Cobalt Atomic
air-acetylene flame of the spectrophotometer. The digestion
Absorption, Direct
procedure summarized in 8.1 is used to determine total
Statistically
recoverable cobalt. The same chelation-extraction procedure
Amount Amount Significant
may be used to determine nickel (Test Methods D 1886),
Added, Found, Bias, % (95 %
chromium (Test Methods D 1687), copper (Test Methods
mg/L mg/L Confidence
Level)
D 1688), iron (Test Methods D 1068), lead (Test Methods
Reagent water, Type II 1.00 1.03 +3.00 Yes
D 3559), and zinc (Test Methods D 1691).
4.00 3.98 −0.50 No
8.00 8.00 0.00 No
Water of choice 1.00 1.01 +1.00 No
18. Interferences
4.00 4.01 +0.25 No
8.00 8.12 +1.50 No
18.1 See Section 9.
19. Apparatus
19.1 All items of apparatus described in Section 10 are
required.
TEST METHOD B—ATOMIC ABSORPTION,
CHELATION-EXTRACTION
20. Reagents and Materials
16. Scope
20.1 Bromphenol Blue Indicator Solution (1 g/L)—Dissolve
0.1 g of bromphenol blue in 100 mL of 50 % ethanol or
16.1 This test method covers the determination of dissolved
2-propanol.
and total recoverable cobalt in most waters and brines. It is the
20.2 Chloroform (CHCl ).
user’s responsibility to ensure the validity of this test method in
a particular matrix. 20.3 Cobalt Solution, Intermediate (1.0 mL = 100 μg Co)—
See 11.2.
16.2 This test method is applicable in the range from 10 to
1000μ g/L of cobalt. The range may be extended to concen- 20.4 Cobalt Solution, Standard (1.0 mL = 1 μg Co
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.