ASTM D3859-15(2023)
(Test Method)Standard Test Methods for Selenium in Water
Standard Test Methods for Selenium in Water
SIGNIFICANCE AND USE
4.1 In most natural waters selenium concentrations seldom exceed 10 μg/L. However, the runoff from certain types of seleniferous soils at various times of the year can produce concentrations as high as several hundred micrograms per litre. Additionally, industrial contamination can be a significant source of selenium in rivers and streams.
4.2 High concentrations of selenium in drinking water have been suspected of being toxic to animal life. Selenium is a priority pollutant and all public water agencies are required to monitor its concentration.
4.3 These test methods determine the dominant species of selenium reportedly found in most natural and wastewaters, including selenities, selenates, and organo-selenium compounds.
SCOPE
1.1 These test methods cover the determination of dissolved and total recoverable selenium in most waters and wastewaters. Both test methods utilize atomic absorption procedures, as follows:
Sections
Test Method A—Gaseous Hydride AAS2, 3
7 – 16
Test Method B—Graphite Furnace AAS
17 – 26
1.2 These test methods are applicable to both inorganic and organic forms of dissolved selenium. They are applicable also to particulate forms of the element, provided that they are solubilized in the appropriate acid digestion step. However, certain selenium-containing heavy metallic sediments may not undergo digestion.
1.3 These test methods are most applicable within the following ranges:
Test Method A—Gaseous Hydride AAS2, 3
1 μg/L to 20 μg/L
Test Method B—Graphite Furnace AAS
2 μg/L to 100 μg/L
These ranges may be extended (with a corresponding loss in precision) by decreasing the sample size or diluting the original sample, but concentrations much greater than the upper limits are more conveniently determined by flame atomic absorption spectrometry.
1.4 The values stated in SI units are to be regarded as standard. The values given in parentheses are mathematical conversions to inch-pound units that are provided for information only and are not considered standard.
1.5 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of regulatory limitations prior to use. For specific hazard statements, see 11.12 and 13.14.
1.6 This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
General Information
Relations
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
Designation: D3859 − 15 (Reapproved 2023)
Standard Test Methods for
Selenium in Water
This standard is issued under the fixed designation D3859; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 1.6 This international standard was developed in accor-
dance with internationally recognized principles on standard-
1.1 These test methods cover the determination of dissolved
ization established in the Decision on Principles for the
and total recoverable selenium in most waters and wastewaters.
Development of International Standards, Guides and Recom-
Both test methods utilize atomic absorption procedures, as
mendations issued by the World Trade Organization Technical
follows:
Barriers to Trade (TBT) Committee.
Sections
2, 3
Test Method A—Gaseous Hydride AAS 7 – 16
2. Referenced Documents
Test Method B—Graphite Furnace AAS 17 – 26
2.1 ASTM Standards:
1.2 These test methods are applicable to both inorganic and
D1129 Terminology Relating to Water
organic forms of dissolved selenium. They are applicable also
D1193 Specification for Reagent Water
to particulate forms of the element, provided that they are
D2777 Practice for Determination of Precision and Bias of
solubilized in the appropriate acid digestion step. However,
Applicable Test Methods of Committee D19 on Water
certain selenium-containing heavy metallic sediments may not
D3370 Practices for Sampling Water from Flowing Process
undergo digestion.
Streams
1.3 These test methods are most applicable within the
D3919 Practice for Measuring Trace Elements in Water by
following ranges:
Graphite Furnace Atomic Absorption Spectrophotometry
2, 3
Test Method A—Gaseous Hydride AAS 1 μg ⁄L to 20 μg ⁄L
D4841 Practice for Estimation of Holding Time for Water
Test Method B—Graphite Furnace AAS 2 μg ⁄L to 100 μg/L
Samples Containing Organic and Inorganic Constituents
These ranges may be extended (with a corresponding loss in
D5673 Test Method for Elements in Water by Inductively
precision) by decreasing the sample size or diluting the original
Coupled Plasma—Mass Spectrometry
sample, but concentrations much greater than the upper limits
D5810 Guide for Spiking into Aqueous Samples
are more conveniently determined by flame atomic absorption
D5847 Practice for Writing Quality Control Specifications
spectrometry.
for Standard Test Methods for Water Analysis
1.4 The values stated in SI units are to be regarded as
standard. The values given in parentheses are mathematical 3. Terminology
conversions to inch-pound units that are provided for informa-
3.1 Definitions:
tion only and are not considered standard.
3.1.1 For definitions of terms used in these test methods,
1.5 This standard does not purport to address all of the refer to Terminology D1129.
safety concerns, if any, associated with its use. It is the
3.2 Definitions of Terms Specific to This Standard:
responsibility of the user of this standard to establish appro- 3.2.1 total recoverable selenium, n—a descriptive term
priate safety, health, and environmental practices and deter-
relating to the selenium forms recovered in the acid-digestion
mine the applicability of regulatory limitations prior to use.
procedure specified in these test methods.
For specific hazard statements, see 11.12 and 13.14.
4. Significance and Use
4.1 In most natural waters selenium concentrations seldom
These test methods are under the jurisdiction of ASTM Committee D19 on
exceed 10 μg/L. However, the runoff from certain types of
Water and are the direct responsibility of Subcommittee D19.05 on Inorganic
seleniferous soils at various times of the year can produce
Constituents in Water.
concentrations as high as several hundred micrograms per litre.
Current edition approved Dec. 1, 2023. Published January 2024. Originally
approved in 1984. Last previous edition approved in 2015 as D3859 – 15. DOI:
10.1520/D3859-15R23.
2 4
Lansford, M., McPherson, E. M., and Fishman, M. J., Atomic Absorption For referenced ASTM standards, visit the ASTM website, www.astm.org, or
Newsletter, Vol 13, No. 4, 1974, pp. 103–105. contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Pollack, E. N., and West, S. J., Atomic Absorption Newsletter, Vol 12, No. 1, Standards volume information, refer to the standard’s Document Summary page on
1973, pp. 6–8. the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
D3859 − 15 (2023)
Additionally, industrial contamination can be a significant TEST METHOD A—GASEOUS HYDRIDE AAS
source of selenium in rivers and streams.
7. Scope
4.2 High concentrations of selenium in drinking water have
7.1 This test method covers the determination of dissolved
been suspected of being toxic to animal life. Selenium is a
and total recoverable selenium in the range from 1 μg ⁄L to
priority pollutant and all public water agencies are required to
20 μg ⁄L. The range may be extended by decreasing the sample
monitor its concentration.
size or diluting the original sample.
4.3 These test methods determine the dominant species of
7.2 This test method has been used successfully with
selenium reportedly found in most natural and wastewaters,
reagent water, natural water, wastewater, and brines. The
including selenities, selenates, and organo-selenium com-
information on precision may not apply to waters of other
pounds.
matrices.
5. Purity of Reagents
8. Summary of Test Method
5.1 Reagent grade chemicals shall be used in all tests.
8.1 The determination consists of the conversion of sele-
Unless otherwise indicated, it is intended that all reagents shall
nium in its various forms to gaseous selenium hydride (hydro-
conform to the specifications of the Committee on Analytical
gen selenide), with the subsequent analysis of the gas by flame
Reagents of the American Chemical Society, where such
AAS.
specifications are available. Other grades may be used, pro-
8.1.1 The conversion consists of (1) decomposition and
vided it is ascertained that the reagent is of sufficiently high
oxidation to selenium (VI), (2) reduction to selenium (IV), and
purity to permit its use without lessening the accuracy of the
(3) final reduction to selenium hydride.
determination.
8.1.2 The absorbance is determined at 196.0 nm in a
hydrogen-argon (air-entrained) flame.
5.2 Purity of Water—Unless otherwise indicated, reference
to water shall be understood to mean reagent water conforming
8.2 Sample concentrations are obtained directly from a
to Specification D1193, Type I. Other reagent water types may
simple concentration versus absorbance calibration curve.
be used provided it is first ascertained that the water is of
8.3 Total recoverable selenium is determined by treating the
sufficiently high purity to permit its use without adversely
entire sample as the procedure indicates, and the dissolved
affecting the bias and precision of the test method. Type II
selenium is determined by treating the filtrate after the sample
water was specified at the time of round robin testing of this
is filtered through a 0.45 μm membrane filter.
test method.
9. Interferences
6. Sampling
9.1 Mercury and arsenic at concentrations greater than
6.1 Collect the samples in accordance with Practices
500 μg ⁄L and greater than 100 μg/L, respectively, may inhibit
D3370. Take the samples in acid-washed TFE-fluorocarbon or
the formation of selenium hydride.
glass bottles. Other types of bottles may be used for sampling,
but should be checked for selenium absorption. The holding 10. Apparatus
time for the samples may be calculated in accordance with
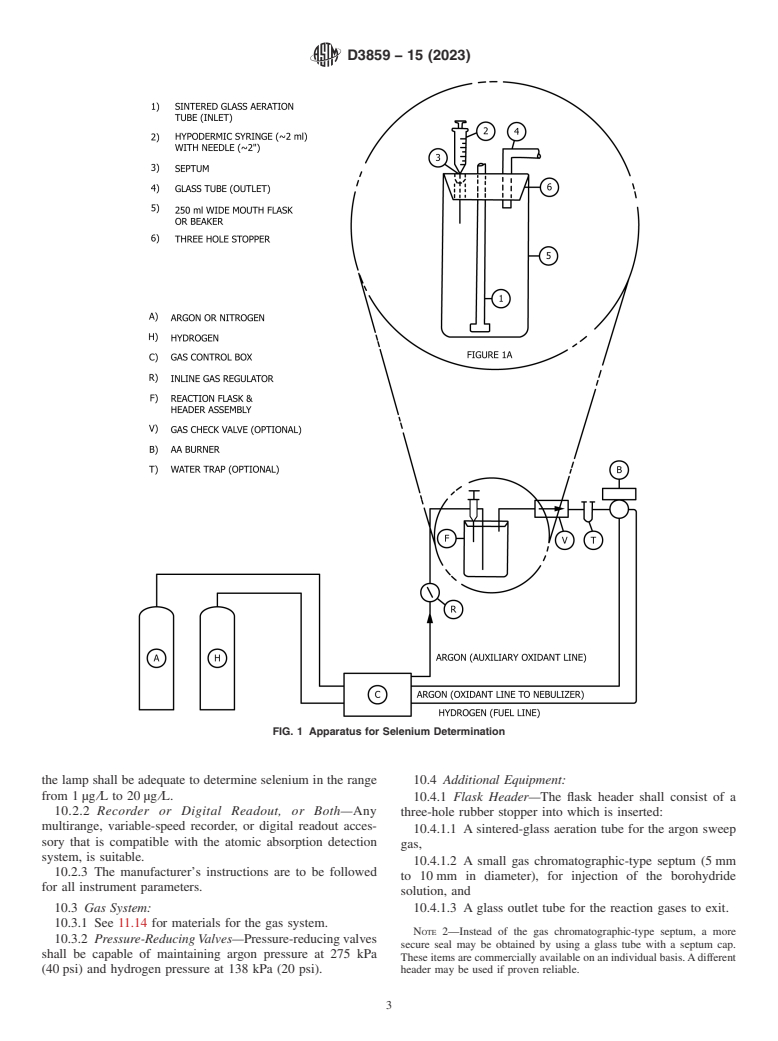
10.1 An apparatus similar to that depicted in Fig. 1, with the
Practice D4841.
components specified in 10.2 – 10.4.8, is recommended for this
test method.
6.2 When determining only dissolved selenium, filter the
sample through a 0.45 μm membrane filter as soon as possible
10.2 Atomic Absorption Spectrophotometer—The instru-
after sampling. Add HNO to the filtrate to bring the pH to
3 ments shall consist of an atomizer and burner, suitable pressure
<2.0.
and flow regulation devices capable of maintaining constant
diluent and fuel pressure for the duration of the test, a selenium
6.3 When determining total recoverable selenium, add
lamp, an optical system capable of isolating the desired
HNO to the unfiltered sample to a pH of <2.0 within 15 min
wavelength, an adjustable slit, a photomultiplier tube or other
of collecting the sample.
photosensitive devices such as a light measuring and amplify-
NOTE 1—Alternatively, the pH may be adjusted in the laboratory if the
ing device, and a readout mechanism for indicating the amount
sample is returned within 14 days. However, acid must be added at least
of absorbed radiation. A background corrector may be used, but
24 h before analysis to dissolve any metals that adsorb to the container
is not absolutely essential.
walls. This could reduce hazards of working with acids in the field when
10.2.1 Selenium Electrodeless Discharge Lamp—The sensi-
appropriate.
tivity of selenium to atomic absorption spectroscopy is gener-
ally improved with this lamp, although some hollow-cathode
lamps produce equivalent results. The intensity and stability of
ACS Reagent Chemicals, Specifications and Procedures for Reagents and
Standard-Grade Reference Materials, American Chemical Society, Washington,
DC. For suggestions on the testing of reagents not listed by the American Chemical
Society, see Analar Standards for Laboratory Chemicals, BDH Ltd., Poole, Dorset, A static system, such as one using a balloon, has been found satisfactory for this
U.K., and the United States Pharmacopeia and National Formulary, U.S. Pharma- purpose. See McFarren, E. F., “New, Simplified Method for Metal Analysis,”
copeial Convention, Inc. (USPC), Rockville, MD. Journal of American Water Works Association, Vol 64, 1972, p. 28.
D3859 − 15 (2023)
FIG. 1 Apparatus for Selenium Determination
the lamp shall be adequate to determine selenium in the range 10.4 Additional Equipment:
from 1 μg ⁄L to 20 μg ⁄L.
10.4.1 Flask Header—The flask header shall consist of a
10.2.2 Recorder or Digital Readout, or Both—Any
three-hole rubber stopper into which is inserted:
multirange, variable-speed recorder, or digital readout acces-
10.4.1.1 A sintered-glass aeration tube for the argon sweep
sory that is compatible with the atomic absorption detection
gas,
system, is suitable.
10.4.1.2 A small gas chromatographic-type septum (5 mm
10.2.3 The manufacturer’s instructions are to be followed
to 10 mm in diameter), for injection of the borohydride
for all instrument parameters.
solution, and
10.3 Gas System: 10.4.1.3 A glass outlet tube for the reaction gases to exit.
10.3.1 See 11.14 for materials for the gas system.
NOTE 2—Instead of the gas chromatographic-type septum, a more
10.3.2 Pressure-Reducing Valves—Pressure-reducing valves
secure seal may be obtained by using a glass tube with a septum cap.
shall be capable of maintaining argon pressure at 275 kPa
These items are commercially available on an individual basis. A different
(40 psi) and hydrogen pressure at 138 kPa (20 psi). header may be used if proven reliable.
D3859 − 15 (2023)
10.4.2 Fittings and Adapters—Stainless steel fittings and 11.10 Selenium Solution, Intermediate (1.00 mL = 10 μg
adapters shall be used to install the reaction-flask header in selenium)—Dilute 5 mL of the selenium stock solution to
series with the auxiliary oxidant line and the burner. Plastic or 500 mL with HCl (1 + 99).
other metals may be substituted if proven acceptable.
11.11 Selenium Solution, Standard (1.00 mL = 0.10 μg
10.4.3 Tubing—Any commercially available plastic tubing
selenium)—Dilute 10 mL of the selenium intermediate solution
that is not susceptible to attack by hydrochloric acid, selenium
to 1000 mL with HCl (1 + 99). Prepare fresh daily and store in
hydride, or other gases from the reaction mixture is acceptable.
a TFE-fluorocarbon or other acceptable container. To minimize
Poly(vinyl chloride) tubing has been found acceptable.
waste only prepare 100 mL of the Selenium Standard Solution.
10.4.4 Gas-Flow Regulator—A suitable in-line gas-flow
11.12 Sodium Borohydride Solution (4 g/100 mL)—
valve shall be used to adjust the flow of argon to the
Dissolve 4 g of sodium borohydride (NaBH ) and 2 g of
reaction-flask header.
sodium hydroxide in water and dilute to 100 mL. Prepare fresh
10.4.5 Water Trap (optional)—Any commercially available
weekly. (Warning—Sodium borohydride reacts strongly with
glass trap suitable to prevent carryover moisture from going to
acids.)
the burner is acceptable.
11.13 Sodium Hydroxide Solution (4 g/L)—Dissolve 4 g of
10.4.6 One-Way Gas Check Valve (optional)—A one-way
sodium hydroxide (NaOH) in water and dilute to 1 L.
check valve can be installed in series with the water trap and
burner to prevent hydrogen from back flowing to the generat-
11.14 Gases:
ing flask whenever samples are changed. However, precaution-
11.14.1 Argon (nitrogen may be used in place of argon)—
ary measures could generally preclude the use of this device,
Standard, commercially available argon is the usual diluent.
since only when the flask header is removed for prolonged
11.14.2 Hydrogen—Standard, commercially available hy-
periods would there be significant hydrogen back flow.
drogen is the usual fuel.
10.4.7 Reaction Flasks, 250 mL spoutless beakers, or their
equivalent, with graduations may be used. Conical and re- 12. Standardization
stricted neck flasks do not perform as reliably as spoutless
12.1 Transfer 0.0 mL, 0.5 mL, 1.0 mL, 2.0 mL, 5.0 mL, and
beakers.
10.0 mL portions of the standard selenium solution
10.4.8 Hypodermic Syringe, 2 mL capacity with a 50 mm
(1.0 mL = 0.10 μg Se) (11.11) to freshly washed 250 mL
needle.
reaction flasks. Adjust the volume to 50 mL with water.
Analyze at least six working standards containing concentra-
11. Reagents and Materials
tions of selenium that bracket the expected sample
concentration, prior to analysis of samples, to calibrate the
11.1 Calcium Chloride Solution (30 g/L)—Commercially
instrument.
purchase or dissolve 30 g of calcium chloride (CaCl ·2H O) in
2 2
water and dilute to 1 L.
12.2 Proceed as directed in 13.3 – 13.15.
11.2 Hydrochloric Acid (sp gr 1.19), concentrated hydro-
12.3 Cali
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.