ASTM G71-81(1998)e1
(Guide)Standard Guide for Conducting and Evaluating Galvanic Corrosion Tests in Electrolytes
Standard Guide for Conducting and Evaluating Galvanic Corrosion Tests in Electrolytes
SCOPE
1.1 This guide is for conducting and evaluating galvanic corrosion tests to characterize the behavior of two dissimilar metals in electrical contact in an electrolyte under low-flow conditions. It can be adapted to wrought or cast metals and alloys.
1.2 This guide covers the selection of materials, specimen preparation, test environment, method of exposure, and method for evaluating the results to characterize the behavior of galvanic couples in an electrolyte. Note-Additional information on galvanic corrosion testing and examples of the conduct and evaluation of galvanic corrosion tests in electrolytes are given in Refs (1) through (7).
1.3 This standard may involve hazardous materials, operations, and equipment. This standard does not purport to address all of the safety problems associated with its use. It is the responsibility of whoever uses this standard to consult and establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn. Contact
ASTM International (www.astm.org) for the latest information.
e1
Designation: G 71 – 81 (Reapproved 1998)
AMERICAN SOCIETY FOR TESTING AND MATERIALS
100 Barr Harbor Dr., West Conshohocken, PA 19428
Reprinted from the Annual Book of ASTM Standards. Copyright ASTM
Standard Guide for
Conducting and Evaluating Galvanic Corrosion Tests in
Electrolytes
This standard is issued under the fixed designation G 71; the number immediately following the designation indicates the year of original
adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A superscript
epsilon (e) indicates an editorial change since the last revision or reapproval.
e NOTE—Section 9 was added editorially in April 1998.
1. Scope 3. Significance and Use
1.1 This guide is for conducting and evaluating galvanic 3.1 Use of this guide is intended to provide information on
corrosion tests to characterize the behavior of two dissimilar the galvanic corrosion of metals in electrical contact in an
metals in electrical contact in an electrolyte under low-flow electrolyte that does not have a flow velocity sufficient to cause
conditions. It can be adapted to wrought or cast metals and erosion-corrosion or cavitation.
alloys. 3.2 This standard is presented as a guide for conducting
1.2 This guide covers the selection of materials, specimen galvanic corrosion tests in liquid electrolyte solutions, both in
preparation, test environment, method of exposure, and method the laboratory and in service environments. Adherence to this
for evaluating the results to characterize the behavior of guide will aid in avoiding some of the inherent difficulties in
galvanic couples in an electrolyte. such testing.
NOTE 1—Additional information on galvanic corrosion testing and
4. Test Specimens
examples of the conduct and evaluation of galvanic corrosion tests in
2 4.1 Material—Test specimens should be manufactured from
electrolytes are given in Refs (1) through (7).
the same material as those used in the service application being
1.3 This standard does not purport to address all of the
modeled. Minor compositional or processing differences be-
safety concerns, if any, associated with its use. It is the
tween materials or between different heats can greatly affect
responsibility of the user of this standard to establish appro-
the results in some cases.
priate safety and health practices and determine the applica-
4.2 Size and Shape:
bility of regulatory limitations prior to use.
4.2.1 The size and shape of the test specimens are dependent
on restrictions imposed by the test location. When determining
2. Referenced Documents
material behavior in the laboratory, it is advisable to use the
2.1 ASTM Standards:
largest specimens permissible within the constraints of the test
G 1 Practice for Preparing, Cleaning, and Evaluating Cor-
equipment. In general, the ratio of surface area to metal volume
rosion Test Specimens
should be large in order to favor maximum corrosion loss per
G 3 Practice for Conventions Applicable to Electrochemical
weight. Sufficient thickness should be employed, however, to
Measurements in Corrosion Testing
minimize the possibility of perforation of the specimens during
G 4 Guide for Conducting Corrosion Coupon Tests in Field
the test exposure. When modeling large components, the size
Applications
of the specimens should be as large as practical. When
G 16 Guide for Applying Statistics to Analysis of Corrosion
modeling smaller components, specimen size should be as
Data
close as possible to that of the application being modeled.
G 31 Practice for Laboratory Immersion Corrosion Testing
Surface area ratio in the test should be identical to the
of Metals
application being modeled. This ratio is defined as the surface
G 46 Guide for Examination and Evaluation of Pitting
area of one member of the couple divided by the surface area
Corrosion
of the other member of the couple. Only the area in contact
with the electrolyte (wetted area) is used in this calculation. In
low-resistivity electrolytes, maintaining proximity between the
This guide is under the jurisdiction of ASTM Committee G-1 on Corrosion of
Metalsand is the direct responsibility of Subcommittee G01.11on Electrochemical
materials being coupled may be more important than maintain-
Measurements in Corrosion Testing.
ing the exact area ratio. Also, with some couples, such as
Current edition approved Nov. 27, 1981. Published January 1982.
copper coupled to aluminum, there may be effects of corrosion
The boldface numbers in parentheses refer to the list of references appended to
the practice. products washing from one electrode to another which may
Annual Book of ASTM Standards, Vol 03.02.
NOTICE:¬This¬standard¬has¬either¬been¬superceded¬and¬replaced¬by¬a¬new¬version¬or¬discontinued.¬
Contact¬ASTM¬International¬(www.astm.org)¬for¬the¬latest¬information.¬
G71
have to be considered in determining specimen placement. greatly change the end results, and replenishment of the
4.2.2 Laboratory tests are normally performed on rectangu- solution should be chosen to be representative of the service
lar plates or on cylinders. When modeling service applications, application. A test system using continuously replenished test
the shapes of the couple members should approximate the electrolytes is often the only solution to this problem.
shapes in the application. Frequently complex shapes are 5.1.3 Periodic measurements of the test environment should
simplified for testing purposes. The shape of the specimen is be made when the test duration in a fixed volume solution is for
more important in electrolytes of low conductivity, where periods of several days or longer. These observations may
voltage drop in the electrolyte is significant. In highly conduc- include temperature, pH, O ,H S, CO ,NH , conductivity, and
2 2 2 3
tive electrolytes, the shapes of the couple members may pertinent metal ion content.
therefore deviate somewhat from the shapes in the application. 5.2 Field Tests—Field testing should be performed in an
4.3 Specimen Preparation: environment similar to the service environment. Periodic
4.3.1 The edges of the test specimens should be prepared so measurements of those environmental variables which could
as to eliminate all sheared or cold-worked metal except that vary with time, such as temperature, dissolved O , and so forth,
cold-working introduced by stamping for identification. Shear- should be made.
ing will, in some cases, cause considerable attack. Therefore,
6. Procedure
specimens having sheared edges should not be used. The edges
6.1 Laboratory Versus Field Testing:
should be finished by machining or polishing. The slight
6.1.1 Galvanic corrosion tests are conducted in the labora-
amount of cold working resulting from machining will not
tory for several purposes: (1) inexpensive screening to reduce
introduce any serious error.
expensive field testing, (2) study of the effects of environmen-
4.3.2 Specimens should be cleaned in accordance with
tal variables, and (3) study of the corrosion accelerating or
Practice G 1, or else the specimen surface condition should be
protective effects of various anode/cathode surface area ratios.
similar to the application being modeled. The metallurgical
6.1.2 The materials proven in the laboratory to be the most
condition of the specimens should be similar to the application
promising should also be tested in the field, since it is
being modeled. In all cases surface contamination, such as dirt,
frequently impossible to duplicate the actual service environ-
grease, oil, and thick oxides, should be removed prior to
ment in the laboratory.
weighing and exposure to the test environment.
6.2 Test Procedure:
4.3.3 The specimen identification system must be one that
6.2.1 Specimens should be electrically joined before expo-
will endure throughout the test period. Edge notches, drilled
sure. There are a number of methods for joining the specimens.
holes, stamped numbers, and tags are some of the methods
Laboratory testing generally employs external electrical con-
used for identification. The identification system must not
nection through wires such as to allow current measurement
induce corrosion attack in any way.
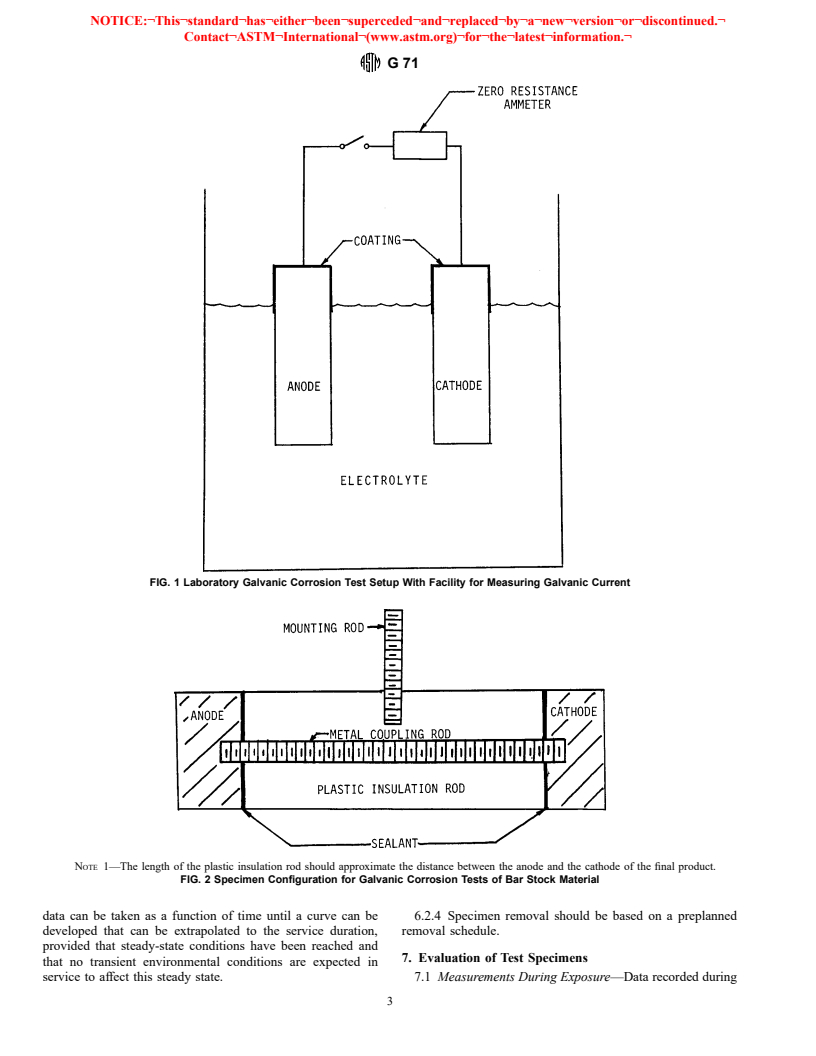
(see Fig. 1). Field tests frequently employ direct contact
4.4 Number of Specimens:
physical bonding by threaded rods as in Fig. 2, soldering,
4.4.1 The number of galvanic couples to be tested will be
brazing, etc. Prime considerations are that the electrical bond to
determined by whether or not one or more periodic specimen
the specimen will not corrode, which could result in decou-
removals are scheduled during the course of the test. As a
pling, that the method of joining will not in itself be a galvanic
minimum, duplicate and preferably triplicate specimens should
couple or introduce other corrosion mechanisms (crevice, and
be tested for any given test period to determine the variability
so forth), and that the resistance of the electrical path be small
in the galvanic corrosion behavior. The effect of the number of
compared to the polarization resistance of the couple materials.
replications on the application of the results is set forth in
Soldering or brazing will prevent the use of mass measure-
Guide G 16.
ments for calculating corrosion rates. A coating may be applied
4.4.2 C
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.