ASTM G3-14(2019)
(Practice)Standard Practice for Conventions Applicable to Electrochemical Measurements in Corrosion Testing
Standard Practice for Conventions Applicable to Electrochemical Measurements in Corrosion Testing
SIGNIFICANCE AND USE
3.1 This practice provides guidance for reporting, displaying, and plotting electrochemical corrosion data and includes recommendations on signs and conventions. Use of this practice will result in the reporting of electrochemical corrosion data in a standard format, facilitating comparison between data developed at different laboratories or at different times. The recommendations outlined in this standard may be utilized when recording and reporting corrosion data obtained from electrochemical tests such as potentiostatic and potentiodynamic polarization, polarization resistance, electrochemical impedance and admittance measurements, galvanic corrosion, and open circuit potential measurements.
SCOPE
1.1 This practice covers conventions for reporting and displaying electrochemical corrosion data. Conventions for potential, current density, electrochemical impedance and admittance, as well as conventions for graphical presentation of such data are included.
1.2 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard. See also 7.4.
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of regulatory limitations prior to use.
1.4 This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
General Information
Relations
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
Designation: G3 − 14 (Reapproved 2019)
Standard Practice for
Conventions Applicable to Electrochemical Measurements
in Corrosion Testing
This standard is issued under the fixed designation G3; the number immediately following the designation indicates the year of original
adoptionor,inthecaseofrevision,theyearoflastrevision.Anumberinparenthesesindicatestheyearoflastreapproval.Asuperscript
epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope between data developed at different laboratories or at different
times. The recommendations outlined in this standard may be
1.1 This practice covers conventions for reporting and
utilized when recording and reporting corrosion data obtained
displaying electrochemical corrosion data. Conventions for
from electrochemical tests such as potentiostatic and potentio-
potential, current density, electrochemical impedance and
dynamic polarization, polarization resistance, electrochemical
admittance, as well as conventions for graphical presentation
impedance and admittance measurements, galvanic corrosion,
of such data are included.
and open circuit potential measurements.
1.2 The values stated in SI units are to be regarded as
standard. No other units of measurement are included in this
4. Sign Convention for Electrode Potential
standard. See also 7.4.
4.1 The Stockholm sign invariant convention is recom-
1.3 This standard does not purport to address all of the
mended for use in reporting the results of specimen potential
safety concerns, if any, associated with its use. It is the
measurements in corrosion testing. In this convention, the
responsibility of the user of this standard to establish appro-
positivedirectionofelectrodepotentialimpliesanincreasingly
priate safety, health, and environmental practices and deter-
oxidizing condition at the electrode in question. The positive
mine the applicability of regulatory limitations prior to use.
direction has also been denoted as the noble direction because
1.4 This international standard was developed in accor-
thecorrosionpotentialsofmostnoblemetals,suchasgold,are
dance with internationally recognized principles on standard-
more positive than the nonpassive base metals. On the other
ization established in the Decision on Principles for the
hand,thenegativedirection,oftencalledtheactivedirection,is
Development of International Standards, Guides and Recom-
associated with reduction and consequently the corrosion
mendations issued by the World Trade Organization Technical
potentials of active metals, such as magnesium. This conven-
Barriers to Trade (TBT) Committee.
tionwasadoptedunanimouslybythe1953InternationalUnion
of Pure and Applied Chemistry as the standard for electrode
2. Referenced Documents
potential (1).
2.1 ASTM Standards:
4.2 In the context of a specimen electrode of unknown
IEEE/ASTM SI 10Standard for Use of the International
potential in an aqueous electrolyte, consider the circuit shown
System of Units (SI) (the Modern Metric System)
in Fig. 1 with a reference electrode connected to the ground
terminal of an electrometer. If the electrometer reads on scale
3. Significance and Use
when the polarity switch is negative, the specimen electrode
3.1 This practice provides guidance for reporting,
potential is negative (relative to the reference electrode).
displaying, and plotting electrochemical corrosion data and
Conversely, if the electrometer reads on scale when polarity is
includes recommendations on signs and conventions. Use of
positive, the specimen potential is positive. On the other hand,
this practice will result in the reporting of electrochemical
if the specimen electrode is connected to the ground terminal,
corrosion data in a standard format, facilitating comparison
the potential will be positive if the meter is on scale when the
polarity switch is negative, and vice versa.
This practice is under the jurisdiction ofASTM Committee G01 on Corrosion
NOTE 1—In cases where the polarity of a measuring instrument is in
ofMetalsandisthedirectresponsibilityofSubcommitteeG01.11onElectrochemi-
doubt, a simple verification test can be performed as follows: connect the
cal Measurements in Corrosion Testing.
measuring instrument to a dry cell with the lead previously on the
Current edition approved May 1, 2019. Published June 2019. Originally
referenceelectrodetothenegativebatteryterminalandtheleadpreviously
approved in 1968. Last previous edition approved in 2014 as G3–14. DOI:
on the specimen electrode to the positive battery terminal. Set the range
10.1520/G0003-14R19.
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Standards volume information, refer to the standard’s Document Summary page on The boldface numbers in parentheses refer to a list of references at the end of
the ASTM website. this standard.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
G3 − 14 (2019)
currentdensitymaybeplottedonlinearorlogarithmicaxes.In
general, logarithmic plots are better suited to incorporation of
widerangesofcurrentdensitydataandfordemonstratingTafel
relationships. Linear plots are recommended for studies in
whichthecurrentdensityorpotentialrangeissmall,orincases
where the region in which the current density changes from
anodic to cathodic is important. Linear plots are also used for
the determination of the polarization resistance R , which is
p
defined as the slope of a potential-current density plot at the
corrosion potential E . The relationship between the polar-
corr
izationresistanceR andthecorrosioncurrentdensityi isas
p corr
follows (2, 3):
d~∆E! b b
a c
5 R 5 (1)
F G
p
di 2.303 b 1b i
~ !
∆E50 a c corr
NOTE 1—The electrode potential of specimen is negative as shown.
where:
FIG. 1 Schematic Diagram of an Apparatus to Measure Electrode
b = anodic Tafel slope,
a
Potential of a Specimen
b = cathodic Tafel slope, and
c
∆E = the difference E−E , where E is the specimen
corr
potential.
switchtoaccommodatethedrycellvoltage.Themeterdeflectionwillnow
show the direction of positive potential.
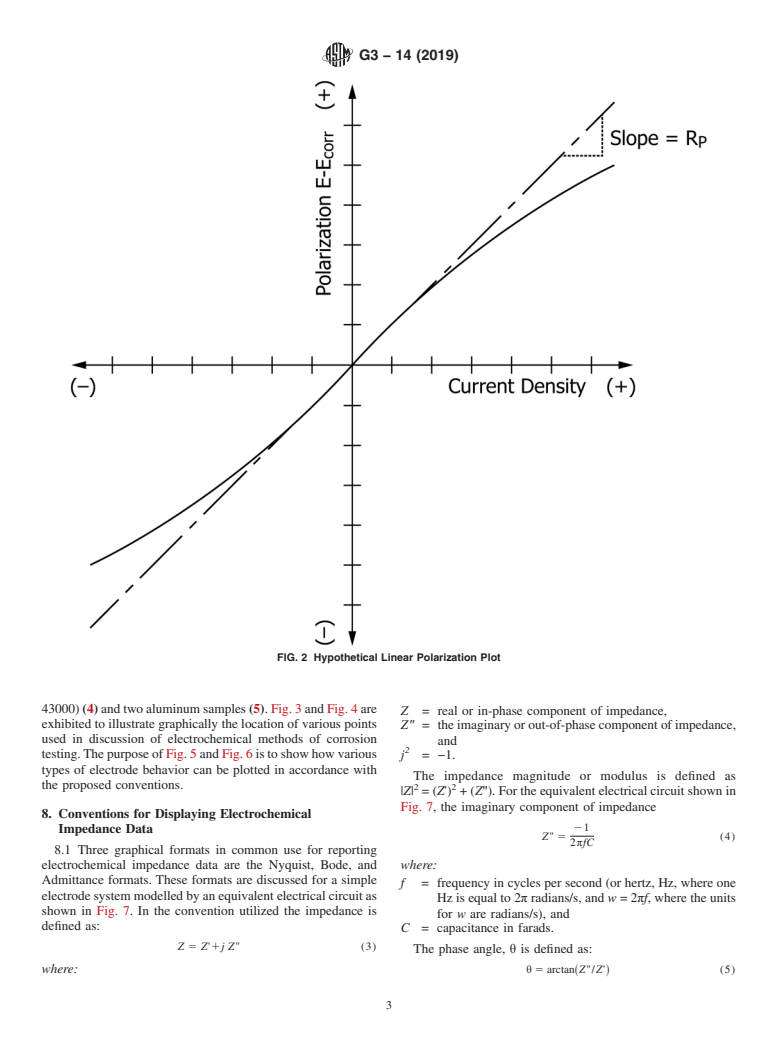
Fig. 2 is a plot of polarization, E−E , versus current
corr
Also, the corrosion potential of magnesium or zinc should be negative
density i (solid line) from which the polarization resistance R
ina1 N NaCl solution if measured against a saturated standard calomel p
has been determined as the slope of the curve at the corrosion
electrode (SCE).
potential E .
corr
5. Sign Convention for Electrode Potential Temperature
7.3 Potential Reference Points—In plots where electrode
Coefficients
potentials are displayed, some indication of the conversion of
5.1 There are two types of temperature coefficients of
the values displayed to both the standard hydrogen electrode
electrode potential: isothermal temperature coefficients and the
scale (SHE) and the saturated calomel electrode scale (SCE) is
thermal coefficients. The sign convention recommended for
recommended if they are known. For example, when electrode
both types of temperature coefficients is that the temperature
potentialisplottedastheordinate,thentheSCEscalecouldbe
coefficient is positive when an increase in temperature pro-
shown at the extreme left of the plot and the SHE scale shown
duces an increase (that is, it becomes more positive) in the
at the extreme right. An alternative, in cases where the
electrode potential. Likewise, the second temperature coeffi-
reference electrode was not either SCE or SHE, would be to
cient is positive when an increase in temperature produces an
show on the potential axis the potentials of these electrodes
increase (that is, it becomes more positive) in the first tem-
against the reference used. In cases where these points are not
perature coefficient.
shown on the plot, an algebraic conversion could be indicated.
For example, in the case of a silver-silver chloride reference
6. Sign Convention for Current and Current Density
electrode(1MKCl),theconversioncouldbeshowninthetitle
6.1 The sign convention in which anodic currents and
box as:
current densities are considered positive and cathodic currents
SCE 5 E 2 0.006V (2)
and current densities are negative is recommended. When the
potential is plotted against the logarithm of the current density,
SHE 5 E10.235V
only the absolute values of the current density can be plotted.
where E represents electrode potential measured against the
In such plots, the values which are cathodic should be clearly silver-silver chloride standard (1 M KCl).
NOTE2—Atableofpotentialsforvariouscommonreferenceelectrodes
differentiated from the anodic values if both are present.
is presented in Appendix X2.
7. Conventions for Displaying Polarization Data
7.4 Units—Therecommendedunitofpotentialisthevolt.In
7.1 Sign Conventions—The standard mathematical practice cases where only small potential ranges are covered, millivolts
or microvolts may be used.The SI units for current density are
for plotting graphs is recommended for displaying electro-
chemical corrosion data. In this practice, positive values are ampere per square metre or milliampere per square centimetre
(IEEE/ASTM SI 10). Still in use are units expressed in
plotted above the origin on the ordinate axis and to the right of
the origin on the abscissa axis. In logarithmic plots, the amperes per square centimetre, and microamperes per square
centimetre.
abscissa value increases from left to right and the ordinate
value increases from bottom to top.
7.5 Sample Polarization Curves—Sample polarization plots
7.2 Current Density-Potential Plots—Auniform convention employing these recommended practices are shown in Figs.
is recommended for plotting current density-potential data, 2-6. Fig. 3 and Fig. 4 are hypothetical curves showing active
namely, plot current density along the abscissa and potential andactive-passiveanodebehavior,respectively.Fig.5andFig.
along the ordinate. In current density potential plots, the 6areactualpolarizationdataforType430stainlesssteel(UNS
G3 − 14 (2019)
FIG. 2 Hypothetical Linear Polarization Plot
43000) (4)andtwoaluminumsamples (5).Fig.3andFig.4are
Z = real or in-phase component of impedance,
exhibitedtoillustrategraphicallythelocationofvariouspoints
Z" = theimaginaryorout-of-phasecomponentofimpedance,
used in discussion of electrochemical methods of corrosion
and
testing.ThepurposeofFig.5andFig.6istoshowhowvarious j = −1.
types of electrode behavior can be plotted in accordance with
The impedance magnitude or modulus is defined as
the proposed conventions. 2 2
|Z| =(Z') +(Z").Fortheequivalentelectricalcircuitshownin
Fig. 7, the imaginary component of impedance
8. Conventions for Displaying Electrochemical
Impedance Data
Z" 5 (4)
2πfC
8.1 Three graphical formats in common use for reporting
electrochemical impedance data are the Nyquist, Bode, and where:
Admittance formats. These formats are discussed for a simple
f = frequency in cycles per second (or hertz, Hz, where one
electrodesystemmodelledbyanequivalentelectricalcircuitas
Hz is equal to 2π radians/s, and w=2πf, where the units
shown in Fig. 7. In the convention utilized the impedance is
for w are radians/s), and
defined as:
C = capacitance in farads.
Z 5 Z'1jZ" (3)
The phase angle, θ is defined as:
where: θ 5 arctan Z"/Z' (5)
~ !
G3 − 14 (2019)
FIG. 3 Hypothetical Cathodic and Anodic Polarization Diagram
FIG. 4 Hypothetical Cathodic and Anodic Polarization Plots for a Passive Anode
The admittance, Y, is defined as
Y' = real or in-phase component of admittance, and
Y" = theimaginaryofout-of-phasecomponentofadmittance.
1/Z 5 Y 5 Y'1jY" (6)
8.2 Nyquist Format (Complex Plane, or Cole-Cole):
where:
G3 − 14 (2019)
FIG. 5 Typical Potentiostatic Anodic Polarization Plot for Type 430 Stainless Steel in 1.0N H SO
2 4
FIG. 6 Typical Polarization Plots for Aluminum Materials in 0.2N NaCl Solution
8.2.1 The real component of impedance is plotted on the 8.2.2 Fig. 8 shows a Nyquist plot for the equivalent circuit
abscissa and the negative of the imaginary component is of Fig. 7. The frequency dependence of the data is not shown
plotted on the ordinate. In this practice positive values of the explicitly on this type of plot. However, the frequency corre-
real component of impedance are plotted to the right of the sponding to selected data points may be directly annotated on
origin parallel to the x axis (abscissa). Negative values of the the Nyquist plot. The magnitude of the appropriate impedance
imaginary component of impedance are plotted vertically from components increases when moving away from the origin of
the origin parallel to the y axis (ordinate). the corresponding axes. Higher frequency data points are
G3 − 14 (2019)
8.3.2 Fig. 9 shows a typical plot for the simple electrical
circuit model of Fig. 7. The magnitude of the high frequency
impedance where the impedance magnitude is independent of
frequency corresponds to R . The difference in magnitude
s
between the low frequency and the high frequency frequency-
independent regions of impedance magnitude corresponds to
R . These resistances are identical to those on the Nyquist
p
format plot shown in Fig. 8.
8.3.3 In the second type of Bode plot, the negative of the
phase angle,−θ, is plotted on the ordinate and the base ten
FIG. 7 Equivalent Electrical Circuit Model for a Simple Corroding
logarithm of the frequency is plotted on the abscissa. In this
Electrode
practice, increasing values of the negative of the phase angle
are plotted in the vertical direction from the origin along the y
axis (ordinate). In this format, a pure capacitive behavior is
plotted as a positive value of 90°. Fig. 10 shows a typical plot
for the simple electrode model shown in Fig. 7.
8.3.4 The units for the frequency on both plots are either
hertz (cycles per second) or radians per second (radians per
second=2π radians per cycle multiplied by the number of
cycles per second). The units of the impedance magnitude are
2 2
ohm·cm . The units ohm·cm are obtained by multiplying the
measured resistance or impedance by the exposed specimen
area. The units of the phase angle are degrees.
8.4 Admittance Format (Complex Plane)—The real compo-
nent of admittance is plotted on the abscissa and the imaginary
component of admittance is plotted on the ordinate. In this
practic
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.