ASTM E2898-14
(Guide)Standard Guide for Risk-Based Validation of Analytical Methods for PAT Applications
Standard Guide for Risk-Based Validation of Analytical Methods for PAT Applications
SIGNIFICANCE AND USE
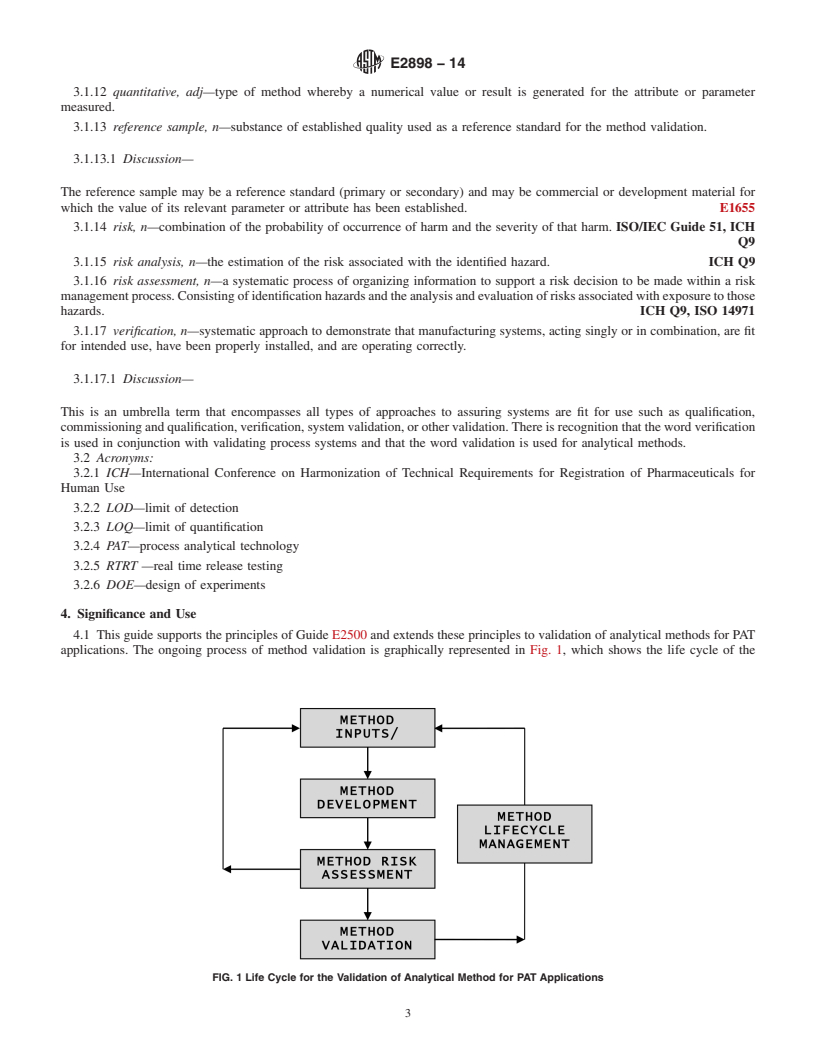
4.1 This guide supports the principles of Guide E2500 and extends these principles to validation of analytical methods for PAT applications. The ongoing process of method validation is graphically represented in Fig. 1, which shows the life cycle of the validation of analytical methods for PAT applications. Prerequisites for validation are the identification of the measurement requirements and development of a method to meet those requirements.
4.2 The method risk assessment also takes into account the stage in the product life cycle at which the measurements are being made and how the resulting data will be used. The integration of these considerations in the risk assessment facilitates the determination of the level of validation necessary to ensure that the method is fit for purpose.
4.3 Changes may occur during the product life cycle necessitating identification of changes to the measurement requirements and method update and revalidation. Procedures should be established to evaluate the continued suitability of the process analytical method.
4.4 Additional informative examples can be found in Practices D3764, D6122, E1655, E1790, E2056, E2617, and E2656 that address validation of methods and models. Other useful standards include ASME BPE2009, ISO 14971, ISO 15839, and USP Acoustic Emission .
SCOPE
1.1 This guide provides an overview to the risk-based validation of process analytical methods under a process analytical technology (PAT) paradigm for pharmaceuticals and biopharmaceuticals and as such includes guidance on assessing risk to product quality from inappropriate method validation.
1.2 This guide builds on existing standards on the topic of validation concentrating on applying such standards to analytical methods for on-line analysis. In particular, it addresses the validation of at-line, on-line, or in-line PAT measurements and covers both API and Drug Product (DP) measurements.
1.3 The definitions of International Conference on Harmonization (ICH) validation parameters (such as specificity, precision, repeatability, etc.) apply; however, the method of demonstrating the validation parameters may vary from that described in ICH and is discussed.
1.4 As consistent with the U.S. Food and Drug Administration (FDA) process validation guidance, this document also briefly covers ongoing assurance that the method remains in a validated state during routine use.
1.5 Equipment and instrument qualification are out of the scope of this guide but will be referenced as inputs to validation of analytical methods for PAT applications.
1.6 The validation of multivariate prediction models is out of scope but will be referenced as inputs to validation of analytical methods for PAT applications.
1.7 Microbiological methods are out of scope.
1.8 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Buy Standard
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: E2898 − 14
Standard Guide for
Risk-Based Validation of Analytical Methods for PAT
1
Applications
This standard is issued under the fixed designation E2898; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 2. Referenced Documents
2
2.1 ASTM Standards:
1.1 This guide provides an overview to the risk-based
validation of process analytical methods under a process D3764 Practice forValidation of the Performance of Process
Stream Analyzer Systems
analytical technology (PAT) paradigm for pharmaceuticals and
biopharmaceuticalsandassuchincludesguidanceonassessing D6122 Practice for Validation of the Performance of Multi-
variate Online,At-Line, and Laboratory Infrared Spectro-
risk to product quality from inappropriate method validation.
photometer Based Analyzer Systems
1.2 This guide builds on existing standards on the topic of
E1655 Practices for Infrared Multivariate Quantitative
validation concentrating on applying such standards to analyti-
Analysis
cal methods for on-line analysis. In particular, it addresses the
E1790 Practice for Near Infrared Qualitative Analysis
validation of at-line, on-line, or in-line PAT measurements and
E2056 Practice for Qualifying Spectrometers and Spectro-
covers both API and Drug Product (DP) measurements.
photometers for Use in Multivariate Analyses, Calibrated
1.3 The definitions of International Conference on Harmo-
Using Surrogate Mixtures
nization (ICH) validation parameters (such as specificity,
E2476 Guide for Risk Assessment and Risk Control as it
precision, repeatability, etc.) apply; however, the method of
Impacts the Design, Development, and Operation of PAT
demonstrating the validation parameters may vary from that
Processes for Pharmaceutical Manufacture
described in ICH and is discussed.
E2500 Guide for Specification, Design, and Verification of
Pharmaceutical and Biopharmaceutical Manufacturing
1.4 As consistent with the U.S. Food and DrugAdministra-
Systems and Equipment
tion (FDA) process validation guidance, this document also
E2617 Practice for Validation of Empirically Derived Mul-
briefly covers ongoing assurance that the method remains in a
tivariate Calibrations
validated state during routine use.
E2629 Guide for Verification of ProcessAnalytical Technol-
1.5 Equipment and instrument qualification are out of the
ogy (PAT) Enabled Control Systems
scope of this guide but will be referenced as inputs to
E2656 PracticeforReal-timeReleaseTestingofPharmaceu-
validation of analytical methods for PAT applications.
tical Water for the Total Organic Carbon Attribute
1.6 The validation of multivariate prediction models is out 3
2.2 ICH Standards:
of scope but will be referenced as inputs to validation of
Q2(R1) Guidance on Validation of Analytical Procedures:
analytical methods for PAT applications.
Text and Methodology
Q7 Good Manufacturing Practice Guide for Active Pharma-
1.7 Microbiological methods are out of scope.
ceutical Ingredients
1.8 This standard does not purport to address all of the
Q9 Quality Risk
safety concerns, if any, associated with its use. It is the
ICH Quality Implementation Working Group Points to
responsibility of the user of this standard to establish appro-
Consider (R2) ICH-Endorsed Guide for ICH Q8/Q9/Q10
priate safety and health practices and determine the applica-
Implementation dated 6 December 2011
bility of regulatory limitations prior to use.
2
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
1
This guide is under the jurisdiction of ASTM Committee E55 on Manufacture contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
ofPharmaceuticalandBiopharmaceuticalProductsandisthedirectresponsibilityof Standards volume information, refer to the standard’s Document Summary page on
Subcommittee E55.01 on Process Understanding and PAT System Management, the ASTM website.
3
Implementation and Practice. Available from International Conference on Harmonisation of Technical
Current edition approved June 1, 2014. Published June 2014. Originally Requirements for Registration of Pharmaceuticals for Human Use (ICH), ICH
approved in 2013. Last previous edition approved in 2013 as E2898 – 13. DOI: Secretariat, c/o IFPMA, 15 ch. Louis-Dunant, P.O. Box 195, 1211 Geneva 20,
10.1520/E2898-14. Switzerland, http://www.ich.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
E2898 − 14
2.3 Other Standards: 3.1.10.1 Discussion—Qualification is part of validation, but
4
ASM
...
This document is not an ASTM standard and is intended only to provide the user of an ASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
Designation: E2898 − 13 E2898 − 14
Standard Guide for
Risk-Based Validation of Analytical Methods for PAT
1
Applications
This standard is issued under the fixed designation E2898; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope
1.1 This guide provides an overview to the risk-based validation of process analytical methods under a process analytical
technology (PAT) paradigm for pharmaceuticals and biopharmaceuticals and as such includes guidance on assessing risk to product
quality from inappropriate method validation.
1.2 This guide builds on existing standards on the topic of validation concentrating on applying such standards to analytical
methods for on-line analysis. In particular, it addresses the validation of at-line, on-line, or in-line PAT measurements and covers
both API and Drug Product (DP) measurements.
1.3 The definitions of International Conference on Harmonization (ICH) validation parameters (such as specificity, precision,
repeatability, etc.) apply; however, the method of demonstrating the validation parameters may vary from that described in ICH
and is discussed.
1.4 As consistent with the U.S. Food and Drug Administration (FDA) process validation guidance, this document also briefly
covers ongoing assurance that the method remains in a validated state during routine use.
1.5 Equipment and instrument qualification are out of the scope of this guide but will be referenced as inputs to validation of
analytical methods for PAT applications.
1.6 The validation of multivariate prediction models is out of scope but will be referenced as inputs to validation of analytical
methods for PAT applications.
1.7 Microbiological methods are out of scope.
1.8 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility
of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory
limitations prior to use.
2. Referenced Documents
2
2.1 ASTM Standards:
D3764 Practice for Validation of the Performance of Process Stream Analyzer Systems
D6122 Practice for Validation of the Performance of Multivariate Online, At-Line, and Laboratory Infrared Spectrophotometer
Based Analyzer Systems
E1655 Practices for Infrared Multivariate Quantitative Analysis
E1790 Practice for Near Infrared Qualitative Analysis
E2056 Practice for Qualifying Spectrometers and Spectrophotometers for Use in Multivariate Analyses, Calibrated Using
Surrogate Mixtures
E2476 Guide for Risk Assessment and Risk Control as it Impacts the Design, Development, and Operation of PAT Processes
for Pharmaceutical Manufacture
E2500 Guide for Specification, Design, and Verification of Pharmaceutical and Biopharmaceutical Manufacturing Systems and
Equipment
1
This guide is under the jurisdiction of ASTM Committee E55 on Manufacture of Pharmaceutical Products and is the direct responsibility of Subcommittee E55.01 on
PAT System Management, Implementation and Practice.
Current edition approved Nov. 1, 2013June 1, 2014. Published December 2013June 2014. Originally approved in 2013. Last previous edition approved in 2013 as E2898
– 13. DOI: 10.1520/E2898-13.10.1520/E2898-14.
2
For referenced ASTM standards, visit the ASTM website, www.astm.org, or contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM Standards
volume information, refer to the standard’s Document Summary page on the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
E2898 − 14
E2617 Practice for Validation of Empirically Derived Multivariate Calibrations
E2629 Guide for Verification of Process Analytical Technology (PAT) Enabled Control Systems
E2656 Practice for Real-time Release Testing of Pharmaceutical Water for the Total Organic Carbon Attribute
3
2.2 ICH Standards:
Q2(R1) Guidance on Validation of Analytical Procedures: Text and Methodology
Q7 Good Manufacturing Practice Guide for Active Pharmaceutical Ingredients
Q9 Quality Risk
ICH Quality Implementation Working Group Points to Consider (R2) ICH-Endorsed Guide for ICH Q8/Q9/Q10 Implementation
dated 6 December 2011
2.3 Other Standards:
4
ASME BPE2009 BioProcessing Equ
...









Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.