ASTM D4413-98(2003)
(Test Method)Standard Test Method for Determination of Ethylene Oxide in Workplace Atmospheres (Charcoal Tube Methodology)
Standard Test Method for Determination of Ethylene Oxide in Workplace Atmospheres (Charcoal Tube Methodology)
SIGNIFICANCE AND USE
Ethylene oxide is a major raw material used in the manufacture of numerous other bulk industrial chemicals as well as a sterilizing agent.
This test method provides a means of evaluating exposure to ethylene oxide in the working environment at the presently recommended exposure guidelines:
5.2.1 OSHA PEL 1 ppm(v) 8-hr TWA.3
5.2.2 ACGIH TLV 1 ppm(v).4
SCOPE
1.1 This test method describes the determination of ethylene oxide (oxirane) in workplace atmospheres using charcoal tube methodology.
1.2 This test method is compatible with low flow rate personal sampling equipment: 10 to 200 mL/min. It can be used for personnel or area monitoring.
1.3 The sampling method develops a time-weighted average (TWA) sample and can be used to determine short-term excursions (STE).
1.4 The applicable concentration range for the TWA sample is from 0.3 to 20 ppm(v).
1.5 The applicable concentration range for the STE sample ranges from 1 to 1000 ppm(v).
1.6 The values stated in SI units shall be regarded as the standard. Inch-pound units are provided for information only.
1.7 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use. (For more specific safety precautionary statements see Section 9 and 10.2.3 and 11.1.3.)
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information.
Designation:D4413–98 (Reapproved 2003)
Standard Test Method for
Determination of Ethylene Oxide in Workplace Atmospheres
(Charcoal Tube Methodology)
This standard is issued under the fixed designation D4413; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 3. Terminology
1.1 Thistestmethoddescribesthedeterminationofethylene 3.1 Definitions:
oxide (oxirane) in workplace atmospheres using charcoal tube 3.1.1 For definitions of terms relating to this test method,
methodology. refer to Terminology D1356 and Practice E355.
1.2 This test method is compatible with low flow rate
4. Summary of Test Method
personal sampling equipment: 10 to 200 mL/min. It can be
4.1 Aknownvolumeofsampleairispassedthroughaglass
used for personnel or area monitoring.
1.3 Thesamplingmethoddevelopsatime-weightedaverage tube packed with activated charcoal. Ethylene oxide is re-
moved from the air stream by adsorption on the charcoal.
(TWA) sample and can be used to determine short-term
excursions (STE). 4.2 A two-section tube containing a front and a backup
section of adsorbent is used to collect the sample. The backup
1.4 The applicable concentration range for theTWAsample
is from 0.3 to 20 ppm(v). section adsorbs vapors that penetrate the front section and is
usedtodetermineifthecollectioncapacityofthetubehasbeen
1.5 The applicable concentration range for the STE sample
ranges from 1 to 1000 ppm(v). exceeded.
4.3 The ethylene oxide is desorbed with carbon disulfide
1.6 The values stated in SI units shall be regarded as the
standard. Inch-pound units are provided for information only. and analyzed with a gas chromatograph equipped with a flame
ionization detector.
1.7 This standard does not purport to address all of the
safety concerns, if any, associated with its use. It is the 4.4 Quantitationisbasedonthecomparisonofpeakheights
or peak areas of the samples with those of standard solutions.
responsibility of the user of this standard to establish appro-
priate safety and health practices and determine the applica- 4.5 Recoveryfactorsaredeterminedbythesametechniques
used for the atmospheric analysis applied to known standards.
bility of regulatory limitations prior to use. (For more specific
safety precautionary statements see Section 9 and 10.2.3 and
5. Significance and Use
11.1.3.)
5.1 Ethylene oxide is a major raw material used in the
2. Referenced Documents
manufacture of numerous other bulk industrial chemicals as
2.1 ASTM Standards: well as a sterilizing agent.
5.2 This test method provides a means of evaluating expo-
D1356 Terminology Relating to Sampling and Analysis of
Atmospheres sure to ethylene oxide in the working environment at the
presently recommended exposure guidelines:
D3686 Practice for Sampling Atmospheres to Collect Or-
ganic Compound Vapors (Activated Charcoal Tube Ad- 5.2.1 OSHA PEL 1 ppm(v) 8-hr TWA.
5.2.2 ACGIH TLV 1 ppm(v).
sorption Method)
E355 Practice for Gas Chromatography Terms and Rela-
6. Interferences
tionships
6.1 Organic components that have the same or nearly the
same retention time as ethylene oxide during gas chromato-
graphic analysis will interfere.
This test method is under the jurisdiction of ASTM Committee D22 on Air
6.2 Other volatile organic compounds in the area where
Quality and is the direct responsibility of Subcommittee D22.04 on Workplace
Atmospheres. samples are taken should be considered.
Current edition approved April 10, 2003. Published June 2003. Originally
´1
approved in 1985. Last previous edition approved in 1998 as D4413–98 . DOI:
10.1520/D4413-98R03. Title 29, Code of Federal Regulation (Section 1910.1047), U.S. Department of
For referenced ASTM standards, visit the ASTM website, www.astm.org, or Labor, revised 49FR 25797 June 22, 1984.
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM “Threshold Limit Values for Chemical Substances and Physical Agents in the
Standards volume information, refer to the standard’s Document Summary page on WorkroomEnvironmentwithIntendedChangesfor1997,”AmericanConferenceof
the ASTM website. Governmental Industrial Hygienists, P.O. Box 1937, Cincinnati, OH 45201.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
D4413–98 (2003)
6.3 Suchinterferencescanbeminimizedbyproperselection 7.1.4 Glasstubesshallbeheldinsuitableprotectiveholders
of gas chromatographic columns.Amass spectrometric detec- to prevent breakage during sampling and to protect workers.
tor can be used to confirm the presence of ethylene oxide.
7.1.5 Polyethylene end caps are used to reseal the charcoal
6.4 Water mists, high humidity, elevated temperatures, and
tubes. Caps must fit tightly to prevent leakage.
high concentrations of other compounds affect adsorption
7.2 Syringes:
efficienciesbyreducingtheadsorptivecapacityofthecharcoal
7.2.1 Gas-Tight Syringe, 1 and 2-mL capacity with a low
for ethylene oxide.
dead-volume needle.
7.2.2 Microlitre Syringes, 10, 100, and 1000-µL or other
7. Apparatus
convenient sizes for making standards.
7.1 Charcoal Sampling Tube:
7.3 Vials, glass, 4, 8, and 12 mL [1, 2, and 3 dram] for
7.1.1 Description—Asampling tube consists of a length of
desorbing samples and holding standards, polyethylene or
glass tubing containing two sections of activated charcoal that
TFE-fluorocarbon-lined screw caps and septum-valve caps.
are held in place by nonadsorbent material and sealed at each
7.4 Styrene Foam Shipping Container,seamlesspolystyrene
end. The front section is retained by a plug of glass wool and
foam container with a minimum wall thickness of 35 mm [1 ⁄8
the back section is retained by a second 2-mm portion of
in.]andapproximately12-L[ ⁄3-ft ]capacity.Othercontainers,
urethane foam or other retainer, such as glass wool. The two
such as vacuum bottles, may be suitable as long as they can
charcoal sections are separated by a 2-mm portion of urethane
maintain the samples at dry-ice temperatures during shipping.
foam. The ends of the tube are flame-sealed (refer to Practice
7.5 Mechanical Shaker, or vibrator that will vigorously
D3686). The back section of the sample tube adsorbs vapors
agitate the desorbing sample.
that penetrate the front section and is used to determine if the
collection capacity of the tube has been exceeded. Instead of a
7.6 Sampling Equipment:
single tube, two tubes in series may be used (see 11.1.12).
7.6.1 Any pump whose flow rate can be accurately deter-
7.1.2 Sampling tubes containing approximately1gof
mined and set at the desired sampling rate is suitable.
activated charcoal are used for sampling ethylene oxide. Two
7.6.2 Asaguideline,suitablepumpsarethosehavingstable
,
types of sampling tubes have been found suitable.
low flow rates, 610% of the set flow rate, within the range of
7.1.2.1 A sampling tube consisting of a glass tube 110-mm
10to100mL/min,forsamplingperiodsofupto8h.Flowrates
long, 10 mm in outside diameter, 8 mm in inside diameter and
up to 200 mL/min can be used for STE (15 min) monitoring.
containing two sections of activated charcoal (Pittsburgh Co-
7.6.3 All sampling pumps shall be carefully calibrated with
conutBase(PCB)20/40mesh), 800and200mg,separatedby
a charcoal tube in the proper sampling position (see Fig.A2.1
a 2-mm section of urethane foam. This tube is capable of
of Practice D3686). The accuracy of determining the total air
sampling 3 to 20 L of air, depending on the environmental
volume sampled should be 100 65%.
conditions, with no or with minimal breakthrough of ethylene
7.6.4 Tubing, rubber or plastic, 6-mm [ ⁄4-in.] bore, about
oxide into the back section (1,2).
90-cm[3-ft]longequippedwithaspringcliptoholdthetubing
7.1.2.2 Asampling tube, consisting of a glass tube 150-mm
and charcoal tube in place on worker’s lapel area.
long, 8 mm in outside diameter, 6 mm in inside diameter and
7.6.4.1 Caution: Sampling tubes shall not be used with
containing two sections of activated charcoal (Columbia JXC,
plastic or rubber tubing upstream of the charcoal. Absorption
20/48mesh), 700and390mg,separatedbya2-mmsectionof
by the tubing may introduce sampling errors.
urethanefoam.Thistubeiscapableofsampling3to8Lofair,
7.7 Gas Chromatograph:
depending on the environmental conditions, with no or with
7.7.1 Gas chromatographs that employ either a flame ion-
minimal breakthrough of ethylene oxide into the back section
ization detector or a detector whose specifications are equiva-
(3).
lent in sensitivity and selectivity should be used. Detectors
7.1.2.3 When sampling under conditions of high humidity,
shall be capable of determining ethylene oxide concentrations
elevated temperatures, or in the presence of high concentra-
of interest with a signal to noise ratio of at least 10 to 1.
tions of other compounds, the lesser volume in 7.1.2.1 and
Suitable detectors are capable of detecting approximately
7.1.2.2 should be used.
−10
1 310 g of ethylene oxide per injection. For example, 3.2
7.1.3 The pressure drop across the charcoal tube should be
µg of ethylene oxide will be collected from a 6-L air sample
no greater than 3.3 kPa [25 mm Hg] at a flow rate of 1000
containing 0.3-ppm ethylene oxide and the use of 5 mL of
mL/min.
desorption solvent will result in a concentration of 0.65 µg of
ethylene oxide per millilitre of CS .
5 2
Activated coconut-shell charcoal (Pittsburgh Coconut Base, 20/40 mesh) has
7.7.2 A gas chromatographic column capable of separating
been found to have adequate adsorption capacity and recovery properties. Prepared
tubes containing activated coconut-shell charcoal (800 mg/200 mg) are available
ethyleneoxidefromothercomponentsisrequired.Anumberof
from a number of sources.
suitable columns have been discussed in the literature (1, 2, 3,
Columbia activated (pelletized) carbon, grade-JXC (20/48 mesh) is no longer
4). Table 1 lists columns and the chromatographic conditions
available. The sole supplier of JXC carbon (700 mg/390 mg) known to the
used for ethylene oxide determination. Table 2 lists the
committee at this time is SKC, Eighty Four, PA. If you are aware of alternate
suppliers, please provide this information to ASTM Headquarters. Your comments
retention lines of some potential interferences for three chro-
will receive careful consideration at a meeting of the responsible technical
matographic columns. Column suitability shall be verified by
committee, which you may attend.
testing two or more columns of dissimilar packings to mini-
Theboldfacenumbersinparenthesesrefertothelistofreferencesattheendof
this test method. mize the possibility of interferences. If the chromatographic
D4413–98 (2003)
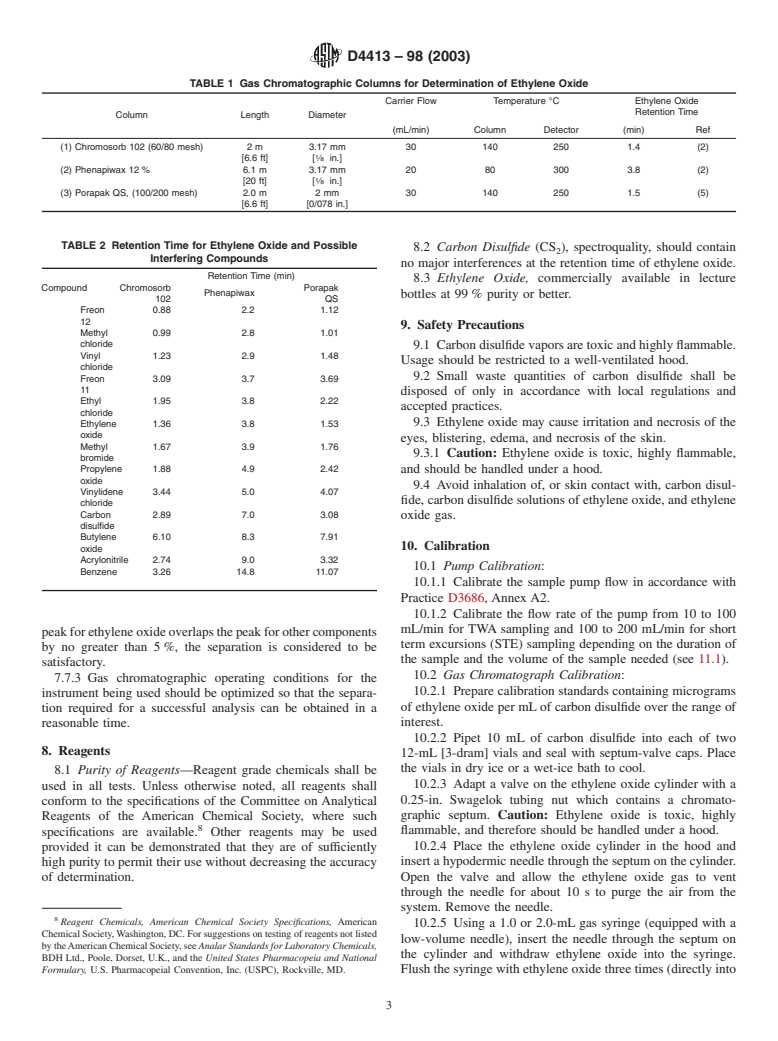
TABLE 1 Gas Chromatographic Columns for Determination of Ethylene Oxide
Carrier Flow Temperature °C Ethylene Oxide
Retention Time
Column Length Diameter
(mL/min) Column Detector (min) Ref
(1) Chromosorb 102 (60/80 mesh) 2 m 3.17 mm 30 140 250 1.4 (2)
[6.6 ft] [ ⁄8 in.]
(2) Phenapiwax 12 % 6.1 m 3.17 mm 20 80 300 3.8 (2)
[20 ft] [ ⁄8 in.]
(3) Porapak QS, (100/200 mesh) 2.0 m 2mm 30 140 250 1.5 (5)
[6.6 ft] [0/078 in.]
TABLE 2 Retention Time for Ethylene Oxide and Possible
8.2 Carbon Disulfide (CS ), spectroquality, should contain
Interfering Compounds
no major interferences at the retention time of ethylene oxide.
Retention Time (min)
8.3 Ethylene Oxide, commercially available in lecture
Compound Chromosorb Porapak
Phenapiwax
bottles at 99% purity or better.
102 QS
Freon 0.88 2.2 1.12
9. Safety Precautions
Methyl 0.99 2.8 1.01
chloride
9.1 Carbondisulfidevaporsaretoxicandhighlyflammable.
Vinyl 1.23 2.9 1.48
Usage should be restricted to a well-ventilated hood.
chloride
9.2 Small waste quantities of carbon disulfide shall be
Freon 3.09 3.7 3.69
disposed of only in accordance with local regulations and
Ethyl 1.95 3.8 2.22
accepted practices.
chloride
Ethylene 1.36 3.8 1.53 9.3 Ethylene oxide may cause irritation and necrosis of the
oxide
eyes, blistering, edema, and necrosis of the skin.
Methyl 1.67 3.9 1.76
9.3.1 Caution: Ethylene oxide is toxic, highly flammable,
bromide
Propylene 1.88 4.9 2.42 and should be handled under a hood.
oxide
9.4 Avoid inhalation of, or skin contact with, carbon disul-
Vinylidene 3.44 5.0 4.07
fide, carbon disulfide solutions of ethylene oxide, and ethylene
chloride
Carbon 2.89 7.0 3.08
oxide gas.
disulfide
Butylene 6.10 8.3 7.91
10. Calibration
oxide
Acrylonitrile 2.74 9.0 3.32
10.1 Pump Calibration:
Benzene 3.26 14.8 11.07
10.1.1 Calibrate the sample pump flow in accordance with
Practice D3686, Annex A2.
10.1.2 Calibrate the flow rate of the pump from 10 to 100
mL/min for TWA sampling and 100 to 200 mL/min for short
peakforethyleneoxideoverlapsthepeakforothercomponents
term excursions (STE) sampling depending on the duration of
by no greater than 5%, the separation is considered to be
the sample and the volume of the sample needed (see 11.1).
satisfactory.
10.2 Gas Chromatograph Calibration:
7.7.3 Gas chromatographic operating conditions for the
10.2.1 Prepare calibration standards containing micrograms
instrument being used should be optimized so that the separa-
of ethylene oxide per mLof carbon disulfide over the range of
tion required for a successful analysis can be obtained in a
interest.
reasonable time.
10.2.2 Pipet 10 mL of carbon disulfide into each of two
8. Reagents
12-mL [3-dram] vials and seal with septum-valve caps. Place
the vials in dry ice or a wet-ice bath to cool.
8.1 Purity of Reagents—Reagent grade chemicals shall be
10.2.3 Adapt a valve on the ethylene oxide cylinder with a
used in all tests. Unless otherwise noted, all reagents shall
0.25-in. Swagelok tubing nut which contains a chromato-
conform to the specifications of the Committee on Analytical
graphic septum. Caution: Ethylene oxide is toxic, highly
Reagents of the American Chemical Society, where such
flammable, and therefore should be handled under a hood.
specifications are available. Other reagents may be used
provided it can be demonstrated that they are of sufficiently 10.2.4 Place the ethylene oxide cylinder in the hood and
insertahypodermicneedlethroughtheseptumonthecylinder.
high purity to permit their use without decreasing the accuracy
of determination. Open the valve and allow the ethylene oxide gas to vent
through the needle for about 10 s to purge the air from the
system. Remove the needle.
Reagent Chemicals, American Chemical Society Specifications, American
10.2.5 Using a 1.0 or 2.0-mL gas syringe (equipped with a
ChemicalSociety,Washington,DC.Fo
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.