ASTM D6208-97(2002)
(Test Method)Standard Test Method for Repassivation Potential of Aluminum and Its Alloys by Galvanostatic Measurement
Standard Test Method for Repassivation Potential of Aluminum and Its Alloys by Galvanostatic Measurement
SCOPE
1.1 A procedure to determine the repassivation potential of aluminum alloy 3003-H14 (UNS A93003) (1) as a measure of relative susceptibility to pitting corrosion by conducting a galvanostatic polarization is described. A procedure that can be used to check experimental technique and instrumentation is described, as well.
1.2 The test method serves as a guide for similar measurement on other aluminum alloys and metals (2-5).
1.3 The values stated in SI units are to be regarded as the standard. Values given in parentheses are for information only.
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: D 6208 – 97 (Reapproved 2002)
Standard Test Method for
Repassivation Potential of Aluminum and Its Alloys by
Galvanostatic Measurement
This standard is issued under the fixed designation D 6208; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope Corrosion Data for Metals for Computerized Database
Input
1.1 A procedure to determine the repassivation potential of
aluminum alloy 3003-H14 (UNSA93003) (1) as a measure of
3. Terminology
relative susceptibility to pitting corrosion by conducting a
3.1 Definitions: An attempt to avoid terminology is made,
galvanostatic polarization is described.Aprocedure that can be
with an explanation provided where applicable. Terms used in
used to check experimental technique and instrumentation is
this test method can be found in Practice G 3 and Terminology
described, as well.
G 15.
1.2 The test method serves as a guide for similar measure-
3.2 Symbols:
ment on other aluminum alloys and metals (2-5).
3.2.1 E —break potential, potential at which the passive
B
1.3 The values stated in SI units are to be regarded as the
aluminum oxide layer breaks down.
standard. Values given in parentheses are for information only.
3.2.2 E —protection potential as measured in this galvano-
G
1.4 This standard does not purport to address all of the
static method, potential at which oxide layer repassivates.
safety concerns, if any, associated with its use. It is the
3.2.3 J—current density, in A/m
responsibility of the user of this standard to establish appro-
priate safety and health practices and determine the applica-
4. Summary of Test Method
bility of regulatory limitations prior to use.
4.1 The test method described is an adaptation of the
method described in FORD Motor Company standards (6).
2. Referenced Documents
4.2 An aluminum alloy specimen is polarized at fixed
2.1 ASTM Standards:
3 current density for 20 min. in a solution of coolant and
D 1193 Specification for Reagent Waters
corrosivewatercontainingchloride.Thepotentialasafunction
D 3585 Specification ASTM Reference Fluid for Coolants
4 of time is recorded.
Tests
4.3 The maximum potential, E reached upon polarization
B
G 3 PracticeforConventionsApplicabletoElectrochemical
5 is determined, as is the minimum potential following the
measurements in Corrosion Testing
maximum potential, E .
G
G 15 Terminology Relating to Corrosion and Corrosion
5 4.4 Visual examination of the specimen may be made using
Testing
Guide G 46 as a guide after disassembly and rinsing.
G 16 Guide forApplying Statistics toAnalysis of Corrosion
Data
5. Significance and Use
G 46 Guide for Examination and Evaluation of Pitting
5.1 This test method is designed to measure the relative
Corrosion
effectiveness of inhibitors to mitigate pitting corrosion of
G 107 Guide for Formats for Collection and Compilation of
aluminum and its alloys, in particular AA3003-H14, rapidly
and reproducibly. The measurements are not intended to
correlate quantitatively with other test method values or with
This test method is under the jurisdiction ofASTM Committee D15 on Engine
susceptibility to localized corrosion of aluminum observed in
Coolants and is the direct responsibility of Subcommittee D15.06 on Glassware
service. Qualitative correlation of the measurements and sus-
Performance Tests.
ceptibility in service has been established (1).
Current edition approved Dec. 10, 1997. Published July 1998.
The boldface numbers in parentheses refer to the list of references at the end of
5.2 The maximum potential reached upon initial polariza-
this standard.
tion, E is a measure of the resistance to breakdown of the
3 B,
Annual Book of ASTM Standards, Vol 11.01.
4 aluminum oxide film. Lower susceptibility to initiation of
Annual Book of ASTM Standards, Vol 15.05.
Annual Book of ASTM Standards, Vol 03.02. pitting corrosion is indicated by a more noble potential (See
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
D 6208 – 97 (2002)
Practice G 3 and Terminology G 15.) This potential, as mea- 6.4 Electrodes:
sured in this test method, is not very sensitive to the inhibitors 6.4.1 Working Electrode (WE)—The working electrode,
present. aluminum test coupon, is cut as 51 3 51 mm (2 in. 3 2 in. )
5.3 The minimum potential, E following the maximum squares from aluminum sheet 2 to 6 mm (1/16 in. to 1/4 in.)
G,
potential is a measure of the protection against continued thick. The standard material is AA3003-H14 (UNS A93003),
pitting corrosion by the inhibitors. Again, a more noble used to develop the precision and bias statements. The coupon
potential indicates better protection. This potential is sensitive is rinsed thoroughly (both sides) with methanol and placed in
to the inhibitors present. a low temperature drying oven. No additional surface prepa-
5.4 Visual examination of the specimens can provide infor- ration is desirable. Prior to testing, a coupon is allowed to cool
mation about subleties of the pitting and inhibition mecha- to room temperature. Then it is clamped to the bottom of the
nisms. Number of pits, pit depth, amount of deposit, and O-ring joint using the matching O-ring (viton or silicone
surface discoloration are some examples of recordable obser- rubber) and clamp. The clamping screw may be tightened to
vations, which can assist evaluation of inhibitor effectiveness. finger tightness, if desired. Excessive tightening must be
5.5 The presence of chloride in the test solution is critical to avoided. This gives an area of 8.72 cm aluminum exposed to
observation of pitting corrosion. Also, a coolant/corrosive the solution.
water solution in which gas bubbles evolve spontaneously on 6.4.2 Auxiliary Electrode (AE)—Ultrafine grade graphite
the aluminum (indicating general corrosion) is unlikely to have rod, 6-8 mm (1/4 in.) in diameter and at least 20 cm (8 in.)
a significant amount of observable pitting corrosion. long. Avoid coarse grades as they can adsorb inhibitors.
6.4.3 Reference Electrode (RE)—The reference electrode
6. Apparatus
can be of any convenient type, for example saturated calomel
(Hg/HgCl)orsilverchloride(Ag/AgCl).Theelectrodemustbe
6.1 General Description—The apparatus for the electro-
in good working order and stable in the solution to be
chemical test consists of a cell, current supply, recorder, and
measured.The reference electrode is placed in Luggin probe to
three electrodes. Fig. 1 is a generalized schematic of the
avoid solution impedance bias. Appendix X2 contains two
arrangement. More specific requirements for each component
suggestions for easily constructed Luggin probes.
are given below.
6.5 Timer—Timer with 1 s resolution out to 30 min.
6.2 Cell—The cell consists of a No.25 O-ring borosilicate
glass joint held vertically using standard laboratory clamps and
7. Preparation of Apparatus
ring stand. The working electrode will be clamped to the
7.1 Assembly—Prior to running tests, assemble the cell and
bottom using the matching O-ring clamp and viton or silicone
electrodes, using an unpreparedAl specimen as the “working”
rubber gasket.
electrode using appropriate clamping. The auxiliary electrode
6.3 Current Supply and Recorder—A constant current sup-
is positioned so that the tip is from 5 to 10 mm from the
ply capable of generating 872 µAcontinuously is required.The
working electrode surface. The Luggin probe is positioned so
recorder must have a high input impedance (> 10 Ohms), be
that the tip is from 1 to 3 mm from the working electrode
capableofrecordingpotentialsof 62VwithmVaccuracy,and
surface. It is most convenient if the clamping arrangement is
have a low gain. These capabilities are typical of commercial
such that this electrode configuration is maintained easily. The
potentiostat/galvanostat instruments connected to either a strip
cell is then removed and Al specimen unclamped.
chart recorder or computer, for experimental control and data
acquisition.The schematic in Fig. 1 shows connections using a
8. Procedure
current supply and mV strip chart recorder, and Fig. X2.1
8.1 A corrosive water containing chloride, sulfate, and
shows a schematic for using a computer and potentiostat/
bicarbonate is prepared by dissolving the following amounts of
galvanostat.
anhydrous salts in distilled or deionized water, ASTM Type II
(see Specification D 1193):
Sodium sulfate 592 mg
Sodium chloride 660 mg
Sodium bicarbonate 552 mg
The solution is made up to a total weight of 1 kg with
distilled or deionized water at 20°C. A 4-kg batch size is
convenient if many tests are to be run, multiply amounts above
byfour.Thiswillgiveasolution,whichis400ppminchloride,
sulfate, and bicarbonate.
8.2 Rinse cell, O-ring, Luggin probe (inside and out),
auxilliary electrode, and reference electrode thoroughly with
Type II water.
8.3 Prepare the aluminum specimen as the working elec-
trode (see 5.4.2). Clamp to cell, using O-ring, and set to one
side.
8.4 Prepare the test solution as 25 vol % of the coolant to be
FIG. 1 Generalized Experimental Set-up tested, 25 vol % of the corrosive water from 6.1, and the
D 6208 – 97 (2002)
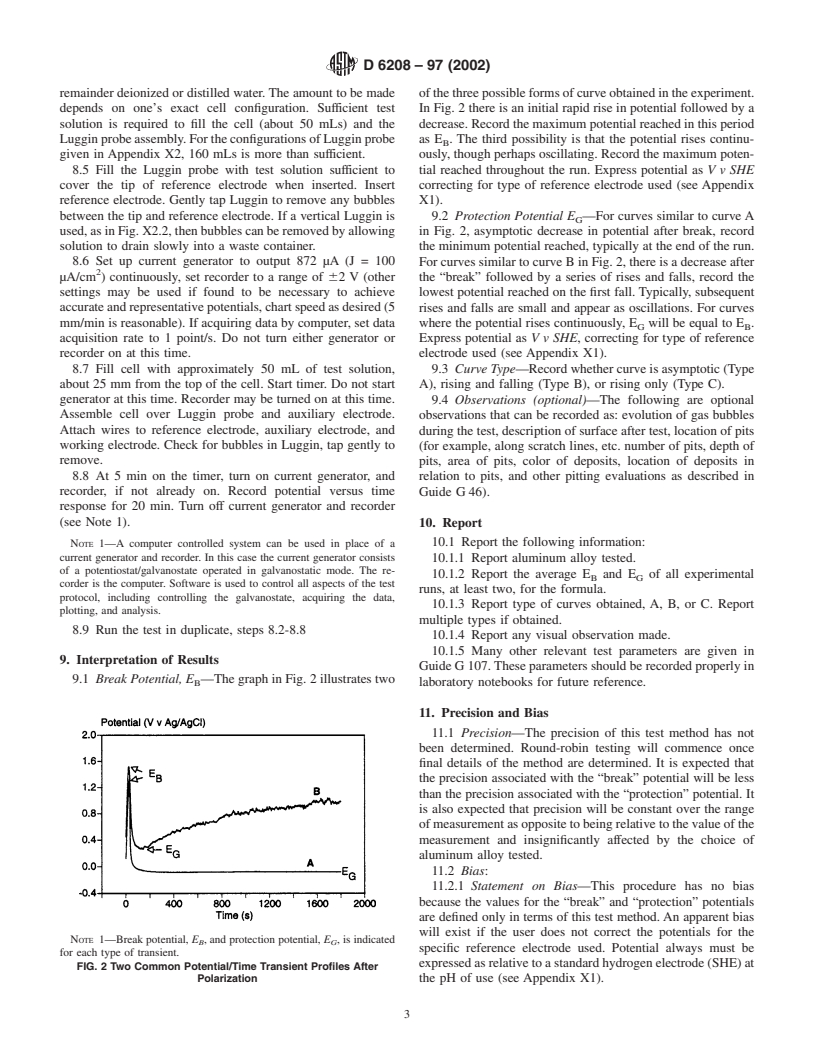
remainder deionized or distilled water. The amount to be made ofthethreepossibleformsofcurveobtainedintheexperiment.
depends on one’s exact cell configuration. Sufficient test In Fig. 2 there is an initial rapid rise in potential followed by a
solution is required to fill the cell (about 50 mLs) and the decrease. Record the maximum potential reached in this period
Lugginprobeassembly.FortheconfigurationsofLugginprobe as E . The third possibility is that the potential rises continu-
B
given in Appendix X2, 160 mLs is more than sufficient. ously, though perhaps oscillating. Record the maximum poten-
8.5 Fill the Luggin probe with test solution sufficient to tial reached throughout the run. Express potential as V v SHE
cover the tip of reference electrode when inserted. Insert correcting for type of reference electrode used (see Appendix
reference electrode. Gently tap Luggin to remove any bubbles X1).
between the tip and reference electrode. If a vertical Luggin is 9.2 Protection Potential E —For curves similar to curve A
G
used,asinFig.X2.2,thenbubblescanberemovedbyallowing in Fig. 2, asymptotic decrease in potential after break, record
solution to drain slowly into a waste container. the minimum potential reached, typically at the end of the run.
8.6 Set up current generator to output 872 µA (J = 100 For curves similar to curve B in Fig. 2, there is a decrease after
µA/cm ) continuously, set recorder to a range of 62 V (other the “break” followed by a series of rises and falls, record the
settings may be used if found to be necessary to achieve lowest potential reached on the first fall. Typically, subsequent
accurateandrepresentativepotentials,chartspeedasdesired(5 rises and falls are small and appear as oscillations. For curves
mm/min is reasonable). If acquiring data by computer, set data
where the potential rises continuously, E will be equal to E .
G B
acquisition rate to 1 point/s. Do not turn either generator or Express potential as V v SHE, correcting for type of reference
recorder on at this time.
electrode used (see Appendix X1).
8.7 Fill cell with approximately 50 mL of test solution, 9.3 Curve Type—Record whether curve is asymptotic (Type
about 25 mm from the top of the cell. Start timer. Do not start
A), rising and falling (Type B), or rising only (Type C).
generator at this time. Recorder may be turned on at this time. 9.4 Observations (optional)—The following are optional
Assemble cell over Luggin probe and auxiliary electrode.
observations that can be recorded as: evolution of gas bubbles
Attach wires to reference electrode, auxiliary electrode, and during the test, description of surface after test, location of pits
working electrode. Check for bubbles in Luggin, tap gently to
(for example, along scratch lines, etc. number of pits, depth of
remove. pits, area of pits, color of deposits, location of deposits in
8.8 At 5 min on the timer, turn on current generator, and
relation to pits, and other pitting evaluations as described in
recorder, if not already on. Record potential versus time Guide G 46).
response for 20 min. Turn off current generator and recorder
(see Note 1).
10. Report
10.1 Report the following information:
NOTE 1—A computer controlled system can be used in place of a
current generator and recorder. In this case the current generator consists
10.1.1 Report aluminum alloy tested.
of a potentiostat/galvanostate operated in galvanostatic mode. The re-
10.1.2 Report the average E and E of all experimental
B G
corder is the computer. Software is used to control all aspects of the test
runs, at least two, for the formula.
protocol, including controlling the galvanostate, acquiring the data,
10.1.3 Report type of curves obtained, A, B, or C. Report
plotting, and analysis.
multiple types if obtained.
8.9 Run the test in duplicate, steps 8.2-8.8
10.1.4 Report any visual observation made.
10.1.5 Many other relevant test parameters are given in
9. Interpretation of Results
Guide G 107.These parameters should be recorded properly in
9.1 Break Potential, E —The graph in Fig. 2 illustrates two
B laboratory notebooks for future reference.
11. Precision and Bias
11.1 Precision—The precision of this test method has not
been determined. Round-robin testing will commence once
final details of the method are determined. It is expected that
the precision associated with the “break” potential will be less
than the precision associated with the “protection” potential. It
is also expected that precision will be constant over the range
ofmeasurementasoppositetobeingrelativetothevalueofthe
measurement and insignificantly affected by the choice of
aluminum alloy tested.
11.2 Bias:
11.2.1 Statement on Bias—This procedure has no bias
because the values for the “break” and “protection” potentials
are defined only in terms of this test method.An apparent bias
will exist if the user does not correct the potentials for the
NOTE 1—Break potential, E , and protection potential, E , is indicated
B G
specific reference electrode used. Potential always must be
for each type of transient.
expressedasrelativetoastandardhydrogenelectrode(SHE)at
FIG. 2 Two Common Potential/Time Transient Profiles After
Polarization the pH of use (see Appendix X1).
D 6208 – 97 (2002)
TABLE 1 Composition of Control
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.