ASTM E1479-99
(Practice)Standard Practice for Describing and Specifying Inductively-Coupled Plasma Atomic Emission Spectrometers
Standard Practice for Describing and Specifying Inductively-Coupled Plasma Atomic Emission Spectrometers
SCOPE
1.1 This practice describes the components of an inductively-coupled plasma optical emission spectrometer that are basic to its operation and to the quality of its performance. It is not the intent of this practice to specify component tolerances or performance criteria, since these are unique for each instrument. The practice does, however, attempt to identify critical factors affecting accuracy, precision, and sensitivity. A prospective user should consult with the vendor before placing an order, to design a testing protocol to demonstrate that the instrument meets all anticipated needs. For more detailed information, consult a publication by Haas, Knisely, Winge, and Fassel.
1.2 This standard does not purport to address all of the safety problems, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use. Specific safety hazard statements are given in Section 9.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation:E1479–99
Standard Practice for
Describing and Specifying Inductively-Coupled Plasma
Atomic Emission Spectrometers
This standard is issued under the fixed designation E 1479; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope 4. Summary of Practice
1.1 This practice describes the components of an 4.1 AnICP-AESisaninstrumentusedtodetermineelemen-
inductively-coupled plasma atomic emission spectrometer tal composition. It typically is comprised of several assemblies
(ICP-AES) that are basic to its operation and to the quality of including a radio-frequency (RF) generator, an impedance
its performance. This practice identifies critical factors affect- matchingnetwork(whererequired),aninductioncoil,aplasma
ing accuracy, precision, and sensitivity. It is not the intent of torch, a plasma ignitor system, a sample introduction system, a
this practice to specify component tolerances or performance light gathering optic, an entrance slit and dispersing element to
criteria, since these are unique for each instrument.Aprospec- sample and isolate wavelengths of light emitted from the
tive user should consult with the vendor before placing an plasma, one or more devices for converting the emitted light
order, to design a testing protocol to demonstrate that the into an electrical current or voltage, one or more analog
instrument meets all anticipated needs. preamplifiers, one or more analog-to-digital converter(s), and a
1.2 This standard does not purport to address all of the dedicated computer with printer (see Fig. 1 ).
safety concerns, if any, associated with its use. It is the 4.1.1 The sample is introduced into a high-temperature
responsibility of the user of this standard to establish appro- (>6000 K) plasma that is formed from the ionization of the gas
priate safety and health practices and determine the applica- stream contained in the torch. The torch is inserted through
bility of regulatory limitations prior to use. Specific safety metal tubing formed into a helix, which is called the load coil.
hazard statements are given in Section 13. Energy is applied to the load coil by means of an RF generator.
4.1.2 Theterminductively-coupledreferstothefactthatthe
2. Referenced Documents
physical phenomenon of induction creates a plasma by trans-
2.1 ASTM Standards:
ferringenergyfromtheloadcoiltothegasstreamthathasbeen
E 135 Terminology Relating to Analytical Chemistry for momentarilypreionizedbyahighvoltageignitorelectrodethat
Metals, Ores, and Related Materials
functions only during plasma ignition.
E 158 Practice for Fundamental Calculations to Convert 4.2 When material passes through the plasma, it is vapor-
Intensities into Concentrations in Optical Emission Spec-
ized, atomized, and many elements are almost completely
trochemical Analysis ionized. Free atoms and ions are excited by collision from their
E 172 Practice for Describing and Specifying the Excitation
ground states. When the excited atoms or ions subsequently
Source in Emission Spectrochemical Analysis decay to a lower energy state, they emit photons, some of
E 416 Practice for Planning and Safe Operation of a Spec-
which pass through the entrance slit of a spectrometer. Each
trochemical Laboratory element emits a unique set of emission lines. Photons of a
E 520 Practice for Describing Photomultiplier Detectors in
desired wavelength may be selected from the ultraviolet and
Emission and Absorption Spectroscopy visible spectra by means of a dispersing element.
4.2.1 Instruments may determine elements either simulta-
3. Terminology
neously or sequentially. The output of the detector generally is
3.1 Definitions—For terminology relating to emission spec-
directed to a preamplifier, an analog-to-digital converter, and a
trometry, refer to Terminology E 135.
computer which measures and stores a value proportional to
the electrical current or voltage generated by the detector(s).
Using blank and known calibration solutions, a calibration
This practice is under the jurisdiction of ASTM Committee E-1 on Analytical
curve is generated for each element of interest.
Chemistry of Metals, Ores, and Related Materials and is the direct responsibility of
Subcommittee E01.20 on Fundamental Practices.
Current edition approved Sept. 10, 1999. Published February 2000. Originally
published as E 1479 – 92. Last previous edition E 1479 – 92.
Annual Book of ASTM Standards, Vol 03.05.
3 4
Annual Book of ASTM Standards, Vol 03.06. Courtesy of PerkinElmer, Inc., 761 Main Ave., Norwalk, CT 06859.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
E1479–99
FIG. 1 Components of Inductively Coupled Plasma
4.2.2 The computer compares the signals arising from the hardware and software, and routine maintenance for at least
various elements in the sample to the appropriate calibration one operator. Training ideally should consist of the basic
curve. The concentrations of more than 70 elements may be operation of the instrument at the time of installation, followed
determined. by an in-depth course one or two months later. Advanced
4.3 Sensitivities (see 12.3) in a simple aqueous solution are courses are also offered at several of the important spectros-
less than one part per million (ppm) for all of these elements, copy meetings that occur throughout the year as well as by
generally less than 10 parts per billion (ppb) for most, and may independent training institutes. Furthermore, several indepen-
even be below 1 ppb for some. dent consultants are available who can provide training, in
4.3.1 Organic liquids may also be used as solvents yielding most cases at the user’s site.
sensitivities that are within an order of magnitude of aqueous
6. Excitation/Radio Frequency Generators
limits for many common organic solvents. Some organic
solvents may afford detection limits similar or even superior to
6.1 Excitation—A specimen is converted into an aerosol
those obtained using aqueous solutions.
entrained in a stream of argon gas and transported through a
4.3.2 Direct sampling of solid materials has been performed
high temperature plasma. The plasma produces excited neutral
successfully by such techniques as spark or laser ablation and
atoms and excited ions. The photons emitted when excited
slurry nebulization. However, these require greater care in the
atoms or ions return to their ground states or lower energy
choice of reference materials and the operation of the sampling
levels are measured and compared to emissions from reference
devices. Solid materials, therefore, are usually dissolved prior
materials of similar composition. For further details see Prac-
to analysis.
tice E 172.
6.2 Radio-Frequency Generator:
5. Significance and Use
6.2.1 An RF generator is used to initiate and sustain the
5.1 This practice describes the essential components of an argon plasma. Commercial generators operate at 27.12 and
inductively-coupled plasma atomic emission spectrometer 40.68 MHz since these frequencies are designated as clear
(ICP-AES). The components include excitation/radio- frequencies by U.S. Federal Communications Committee
frequency generators, sample introduction systems, spectrom- (FCC) regulations. Generators typically are capable of produc-
eters, detectors, and signal processing and displays. This ing 1.0 to 2.0 kW for the 27.12 MHz generator and 1.0 to 2.3
description allows the user or potential user to gain a cursory kW for the 40.68 MHz system.
understanding of an ICP-AES system. This practice also 6.2.2 Generators more powerful than 2.5 kW are of limited
provides a means for comparing and evaluating various sys- practical analytical utility and are not commercially marketed
tems, as well as understanding the capabilities and limitations with ICPspectrometers. The power requirements are related to
of each instrument. torch geometry and types of samples to be analyzed. Refer to
5.2 Training—The vendor should provide training in safety, Practice E 172 for details. More power (typically 1.5 to 2 kW
basic theory of ICP spectrochemical analysis, operations of for a 27.12 MHz system utilizing a 20-mm outside diameter
E1479–99
torch and 1.2 to 1.7 kWfor a 40.68 MHz generator) is required 7.2.2 Some nebulizers, designated as self-aspirating pneu-
for analyzing samples dissolved in organic solvents than is matic nebulizers, operating on the Venturi principle, create a
needed for aqueous solutions (approximately 1.0 kW). Less partial vacuum to force liquid up a capillary tube into the
power is required for small diameter torches (for example, 650 nebulizer. Precision of operation may be improved if a peri-
to 750 W for a 13-mm outside diameter torch). staltic pump controls the solution flow rate.
6.3 Load Coil: 7.2.3 Other nebulizers require an auxiliary device, such as a
6.3.1 Acoil made from copper (or another metal or an alloy
peristaltic pump, to drive solution to the nebulizer. Generally,
with similar electrical properties) is used to transmit power pump-fed nebulizers are more tolerant of high levels of
from the generator to the plasma torch (see 7.6). A typical
dissolved solids and much less affected by suspended solids
designconsistsofatwo-tosix-turncoilofabout1-in.(25-mm) and viscosity variations.
diameter, made from ⁄8-in. (3-mm) outside diameter and
7.2.4 If fluoride is present in solutions to be analyzed, it is
⁄16-in. (1.6-mm) inside diameter copper tubing (though larger necessary to employ a nebulizer fabricated from hydrofluoric
tubing is used with two-turn coils). The tubing is fitted with
acid (HF)-resistant materials (see 7.4.1.). It is possible to use
ferrules or similar devices to provide a leak-free connection to theHF-resistantnebulizerformostothertypesofsolutions,but
a coolant, either recirculated by a pump or fed from a
sensitivity and precision may be degraded. An HF-resistant
municipal water supply. Argon gas blown through the coil has
nebulizer may be more expensive to acquire and repair, and
been used to cool other load coils.
require greater operator proficiency and training than other
6.3.2 Thehighpowerconductedbythecoilcanleadtorapid
nebulizers.
oxidation, surface metal vaporization, RF arc-over and even
7.3 Self-Aspirating or Non-Pump-Fed Nebulizers:
melting if the coil is not cooled continuously.
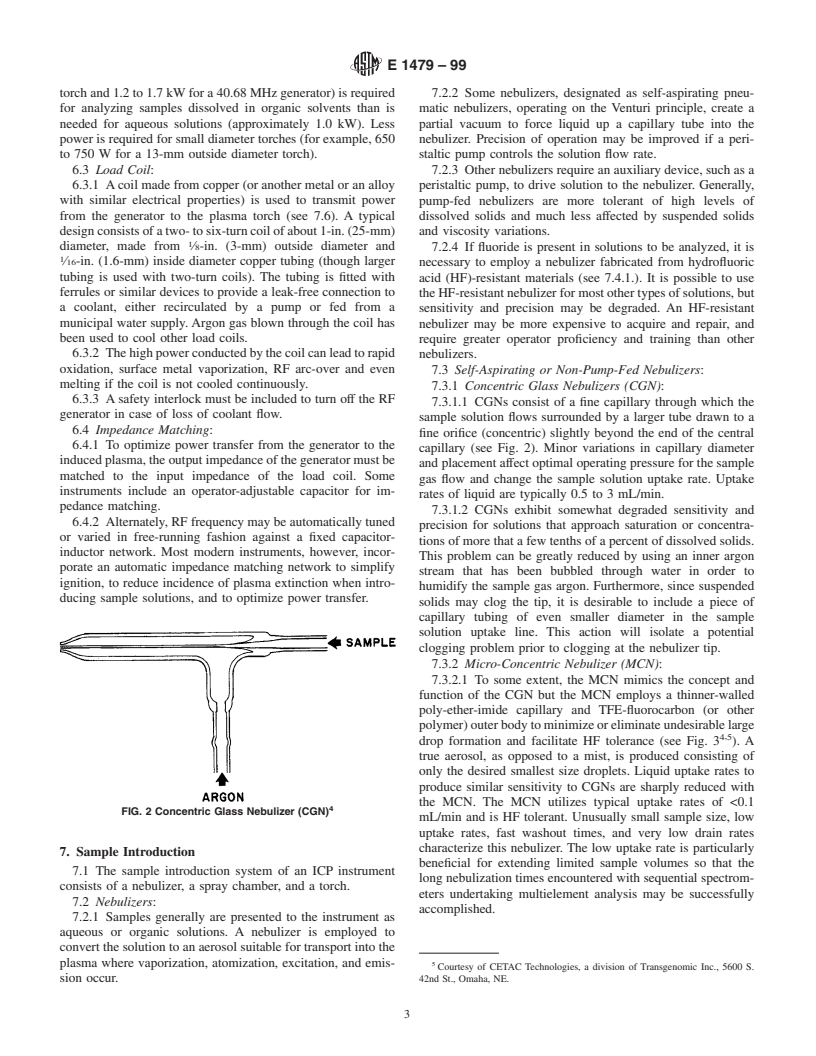
7.3.1 Concentric Glass Nebulizers (CGN):
6.3.3 Asafety interlock must be included to turn off the RF
7.3.1.1 CGNs consist of a fine capillary through which the
generator in case of loss of coolant flow.
sample solution flows surrounded by a larger tube drawn to a
6.4 Impedance Matching:
fine orifice (concentric) slightly beyond the end of the central
6.4.1 To optimize power transfer from the generator to the
capillary (see Fig. 2). Minor variations in capillary diameter
inducedplasma,theoutputimpedanceofthegeneratormustbe
and placement affect optimal operating pressure for the sample
matched to the input impedance of the load coil. Some
gas flow and change the sample solution uptake rate. Uptake
instruments include an operator-adjustable capacitor for im-
rates of liquid are typically 0.5 to 3 mL/min.
pedance matching.
7.3.1.2 CGNs exhibit somewhat degraded sensitivity and
6.4.2 Alternately, RF frequency may be automatically tuned
precision for solutions that approach saturation or concentra-
or varied in free-running fashion against a fixed capacitor-
tions of more that a few tenths of a percent of dissolved solids.
inductor network. Most modern instruments, however, incor-
This problem can be greatly reduced by using an inner argon
porate an automatic impedance matching network to simplify
stream that has been bubbled through water in order to
ignition, to reduce incidence of plasma extinction when intro-
humidify the sample gas argon. Furthermore, since suspended
ducing sample solutions, and to optimize power transfer.
solids may clog the tip, it is desirable to include a piece of
capillary tubing of even smaller diameter in the sample
solution uptake line. This action will isolate a potential
clogging problem prior to clogging at the nebulizer tip.
7.3.2 Micro-Concentric Nebulizer (MCN):
7.3.2.1 To some extent, the MCN mimics the concept and
function of the CGN but the MCN employs a thinner-walled
poly-ether-imide capillary and TFE-fluorocarbon (or other
polymer)outerbodytominimizeoreliminateundesirablelarge
4,5
drop formation and facilitate HF tolerance (see Fig. 3 ). A
true aerosol, as opposed to a mist, is produced consisting of
only the desired smallest size droplets. Liquid uptake rates to
produce similar sensitivity to CGNs are sharply reduced with
the MCN. The MCN utilizes typical uptake rates of <0.1
FIG. 2 Concentric Glass Nebulizer (CGN)
mL/min and is HF tolerant. Unusually small sample size, low
uptake rates, fast washout times, and very low drain rates
characterize this nebulizer. The low uptake rate is particularly
7. Sample Introduction
beneficial for extending limited sample volumes so that the
7.1 The sample introduction system of an ICP instrument
long nebulization times encountered with sequential spectrom-
consists of a nebulizer, a spray chamber, and a torch.
eters undertaking multielement analysis may be successfully
7.2 Nebulizers:
accomplished.
7.2.1 Samples generally are presented to the instrument as
aqueous or organic solutions. A nebulizer is employed to
convert the solution to an aerosol suitable for transport into the
plasma where vaporization, atomization, excitation, and emis-
Courtesy of CETAC Technologies, a division of Transgenomic Inc., 5600 S.
sion occur. 42nd St., Omaha, NE.
E1479–99
FIG. 5 Grid Nebulizer
nebulizer is shown in Fig. 5. The grid nebulizer may be
employed to analyze fluoride-containing solutions, but an
HF-resistant spray chamber and torch must also be used.
7.4.2 Babington, Modified Babington or V-Groove Nebu-
,
FIG. 3 Micro-Concentric Nebulizer (MCN)
lizer:
7.4.2.1 These nebulizers operate by passing an argon gas
7.3.2.2 The initial purchase cost is higher for the MCN than
flow through a falling film of flowing analyte solution. The
for the CGN but the cost may be offset by a substantial
falling film is typically guided by a shallow groove or channel
reduction in recurring hazardous waste disposal cost (for
to a pressurized argon orifice. Film thickness varies with
example, heavy metal salts, mineral acids, etc.). This disposal
channel depth
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.