ASTM F366-82(2000)
(Specification)Standard Specification for Fixation Pins and Wires
Standard Specification for Fixation Pins and Wires
SCOPE
1.1 This specification covers functional dimensions for fixation pins and wires.
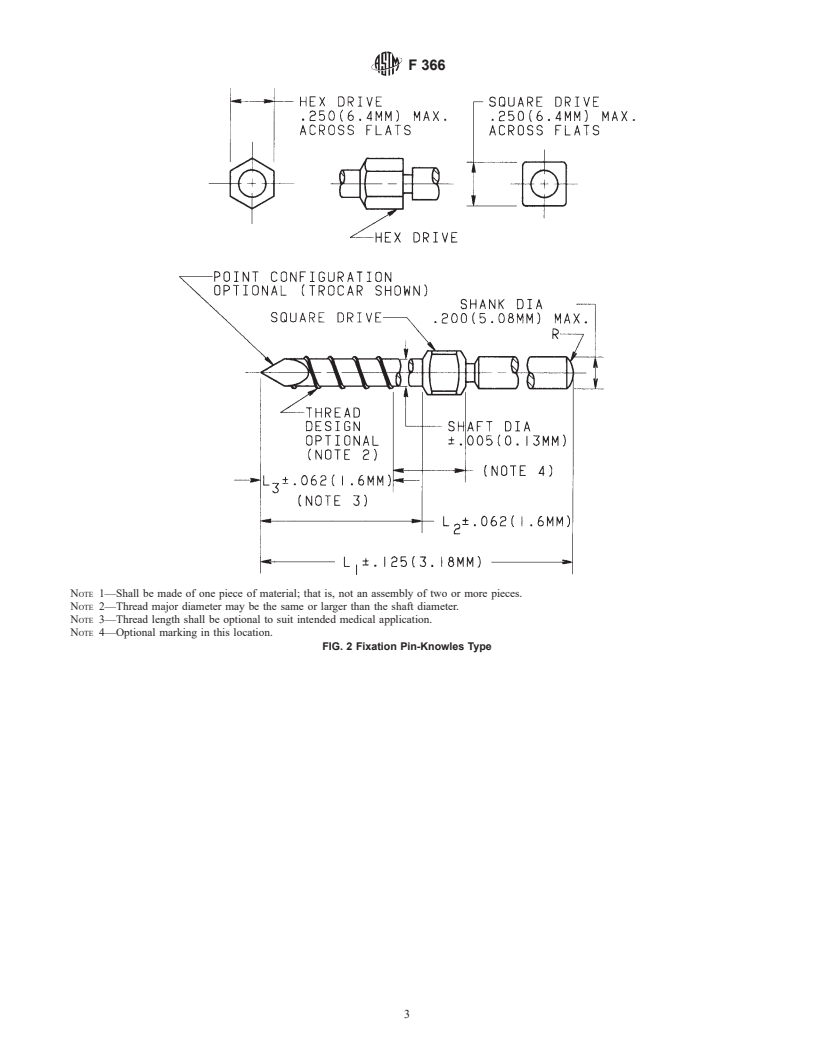
1.2 In recognition of many broad and varied uses of such pins and wires, many options are included. A variety, but not necessarily all, of the options are illustrated in Figs. 1-3.
1.3 The values stated in inch-pound units are to be regarded as the standard.
1.4 The values given in parentheses are provided for information only.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: F 366 – 82 (Reapproved 2000)
Standard Specification for
Fixation Pins and Wires
This standard is issued under the fixed designation F 366; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
This standard has been approved for use by agencies of the Department of Defense.
1. Scope Molybdenum-Tungsten-Iron Alloy for Surgical Implant
Applications
1.1 This specification covers functional dimensions for
fixation pins and wires.
3. Materials
1.2 In recognition of many broad and varied uses of such
3.1 Fixation pins and wires shall be fabricated from material
pins and wires, many options are included. A variety, but not
conforming to one of the following ASTM Specifications:
necessarily all, of the options are illustrated in Figs. 1-3.
F 55, F 67, F 90, F 136, F 138, F 562, or F 563.
1.3 The values stated in inch-pound units are to be regarded
as the standard.
4. Performance Requirements
1.4 The values given in parentheses are provided for infor-
4.1 Factors considered to be important, but for which values
mation only.
and test methods have not been established, are bending
strength, fatigue strength, breaking strength (Knowles Type
2. Referenced Documents
only), torsion strength, and ductility.
2.1 ASTM Standards:
F 55 Specification for Stainless Steel Bar and Wire for
5. Dimensions and Characteristics
Surgical Implants
5.1 Fixation pins and wires shall be fabricated in accordance
F 67 Specification for Unalloyed Titanium for Surgical
3 with the dimensions illustrated in Figs. 1-4.
Implant Applications
5.2 Fixation pins and wires shall have surfaces prepared and
F 86 Practice for Surface Preparation and Marking of Me-
3 marked in accordance with Practice F 86.
tallic Surgical Implants
5.2.1 Optional marking on the fixation pins and wires shall
F 90 Specification for Wrought Cobalt-Chromium-
3 identify the manufacturer or distributor.
Tungsten-Nickel Alloy for Surgical Implant Applications
F 136 Specification for Wrought Titanium 6A1-4V ELI
6. Packaging and Labeling
Alloy for Surgical Implant Applications
6.1 Packaging shall be adequate to protect the fixation pins
F 138 Specification for Stainless Steel Bar and Wire for
and wires during shipment.
Surgical Implants (Special Quality)
6.2 Labeling for fixation pins and wires shall include:
F 562 Specification for Wrought Cobalt-35 Nickel-20
6.2.1 Product name,
Chromium-10 Molybdenum Alloy for Surgical Implant
6.2.2 Size, on the immediate container,
Applications
6.2.2.1 Length,
F 563 Specification for Wrought Cobalt-Nickel-Chromium-
6.2.2.2 Diameter (if round) or cross-sectional size (if square
of hexagonal), that is, ⁄4 in. (6.4 mm) square, and
1 6.2.3 ASTM material specification Designation number.
This specification is under the jurisdiction of ASTM Committee F-4 on Medical
and Surgical Materials and Devices, and is the direct responsibility of Subcommittee
7. Keywords
F04.21 on Osteosynthesis.
Current edition approved Aug. 27, 1982. Published February 1983. Originally
7.1 fixation materials; flexible surgical wire; orthopaedic
published as F 366-73. Last previous edition F 366-73.
2 medical devices; wire-surgical implants
Discontinued—See 1991 Annual Book of ASTM Standards, Vol 13.01.
Annual Book of ASTM Standards, Vol 13.01.
Copyright ©
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.