ASTM E1698-95
(Practice)Standard Practice for Testing Electrolytic Conductivity Detectors (ELCD) Used in Gas Chromatography
Standard Practice for Testing Electrolytic Conductivity Detectors (ELCD) Used in Gas Chromatography
SCOPE
1.1 This practice covers testing the performance of an electrolytic conductivity detector (ELCD) used as the detection component of a gas chromatographic system.
1.2 This practice is directly applicable to electrolytic conductivity detectors that perform a chemical reaction on a given sample over a nickel catalyst surface under oxidizing or reducing conditions and employ a scrubber, if needed, to remove interferences, deionized solvent to dissolve the reaction products, and a conductivity cell to measure the electrolytic conductivity of ionized reaction products.
1.3 This practice covers the performance of the detector itself, independently of the chromatographic column, in terms that the analyst can use to predict overall system performance when the detector is coupled to the column and other chromatographic system components.
1.4 For general gas chromatographic procedures, Practice E260 should be followed except where specific changes are recommended herein for the use of an electrolytic conductivity detector. For definitions of gas chromatography and its various terms see Practice E355.
1.5 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
Designation: E 1698 – 95
Standard Practice for
Testing Electrolytic Conductivity Detectors (ELCD) Used in
Gas Chromatography
This standard is issued under the fixed designation E 1698; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope tions, including geometry, gas and solvent flow rates, and
temperatures. It should be noted that to specify a detector’s
1.1 This practice covers testing the performance of an
capability completely, its performance should be measured at
electrolytic conductivity detector (ELCD) used as the detection
several sets of conditions within the useful range of the
component of a gas chromatographic system.
detector. The terms and tests described in this practice are
1.2 This practice is directly applicable to electrolytic con-
sufficiently general so that they may be used at whatever
ductivity detectors that perform a chemical reaction on a given
conditions may be chosen for other reasons.
sample over a nickel catalyst surface under oxidizing or
3.2 Linearity and speed of response of the recorder used
reducing conditions and employ a scrubber, if needed, to
should be such that it does not distort or otherwise interfere
remove interferences, deionized solvent to dissolve the reac-
with the performance of the detector. Effective recorder re-
tion products, and a conductivity cell to measure the electro-
sponse should be sufficiently fast so that it can be neglected in
lytic conductivity of ionized reaction products.
sensitivity of measurements. If additional amplifiers are used
1.3 This practice covers the performance of the detector
between the detector and the final readout device, their
itself, independently of the chromatographic column, in terms
characteristics should also first be established.
that the analyst can use to predict overall system performance
when the detector is coupled to the column and other chro-
4. Principles of Electrolytic Conductivity Detectors
matographic system components.
4.1 The principle components of the ELCD are represented
1.4 For general gas chromatographic procedures, Practice
in Fig. 1 and include: a control module, a reactor assembly,
E 260 should be followed except where specific changes are
and, a cell assembly.
recommended herein for the use of an electrolytic conductivity
4.1.1 The control module typically will house the detector
detector. For definitions of gas chromatography and its various
electronics that monitor or control, or both, the solvent flow,
terms see Practice E 355.
reaction temperatures, and the conductivity detector cell. It can
1.5 This standard does not purport to address all of the
be functionally independent of the gas chromatography or, in
safety concerns, if any, associated with its use. It is the
some varieties, designed into the functional framework of the
responsibility of the user of this standard to establish appro-
gas chromatograph. However, the reactor and cell assemblies
priate safety and health practices and determine the applica-
are designed for specific models of gas chromatographs so it is
bility of regulatory limitations prior to use.
important the proper components be assembled on the appro-
2. Referenced Documents priate chromatographic equipment.
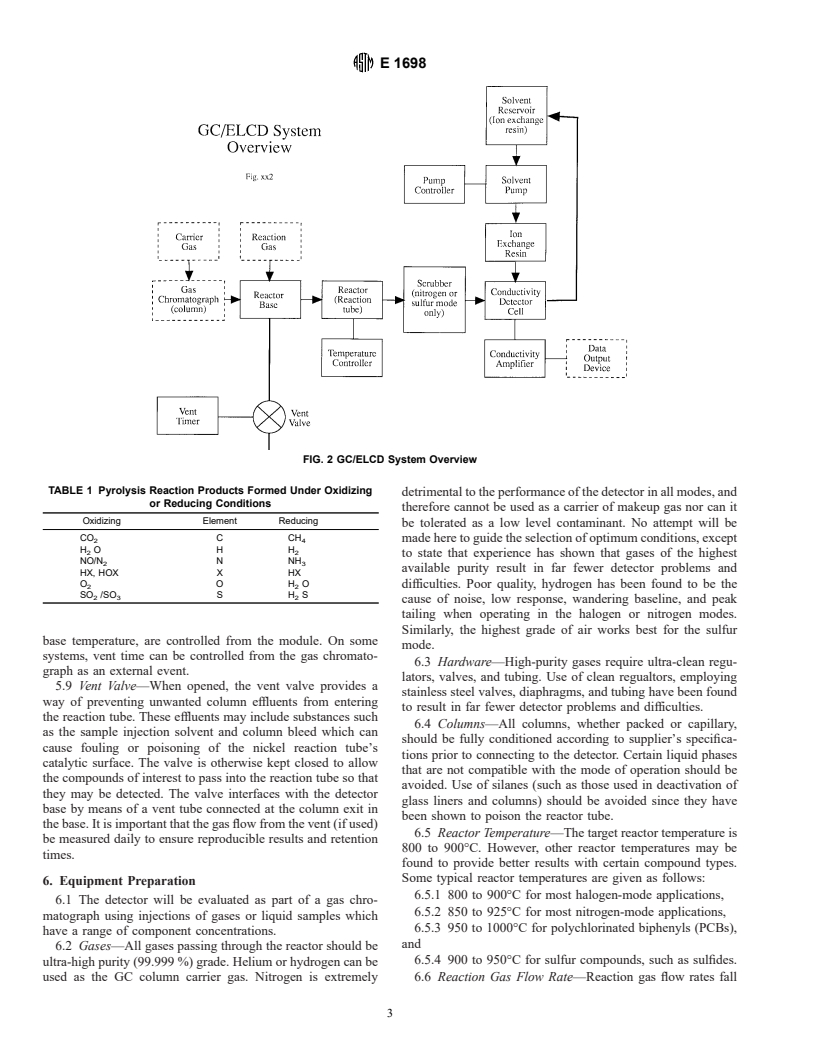
4.2 Fig. 2 is a block diagram representation of the GC/
2.1 ASTM Standards:
2 ELCD system. The electrolytic conductivity detector detects
E 260 Practice for Packed Column Gas Chromatography
compounds by pyrolyzing those compounds in a heated nickel
E 355 Practice for Gas Chromatography Terms and Rela-
2 catalyst (housed in the reactor), removing interfering reaction
tionships
products with a scrubber (if needed), dissolving the reaction
3. Significance and Use products in a suitable solvent, and measuring the change in
electrical conductivity using a conductivity detector cell. Other
3.1 Although it is possible to observe and measure each of
suitable non-catalystic reaction tubes can be used for more
the several characteristics of the ELCD under different and
selective response characteristics. Using the conditions set
unique conditions, in particular its different modes of selectiv-
forth in this practice, halogen (Cl, Br, I, F) compounds,
ity, it is the intent of this practice that a complete set of detector
nitrogen compounds, and sulfur compounds can be measured
specifications should be obtained at the same operating condi-
selectively, even in the presence of each other.
4.3 The electrolytic conductivity detector pyrolyzes com-
This practice is under the jurisdiction of ASTM Committee E13 on Molecular
pounds as they elute from the chromatographic column through
Spectroscopy and is the direct responsibility of Subcommittee E13.19 on Chroma-
tography. a hot nickel reaction tube. Halogen and nitrogen compounds
Current edition approved March 15, 1995. Published July 1995.
are detected under reducing conditions while sulfur compounds
Annual Book of ASTM Standards, Vol 14.02.
Copyright © ASTM, 100 Barr Harbor Drive, West Conshohocken, PA 19428-2959, United States.
E 1698
all aspects of the different detector designs available but rather
to consider one generalized design as an example and recog-
nize that variants may exist.
5.2 Detector Base—The base extends into the gas chroma-
tography oven and permits an inert low dead volume interface
of the column to the reactor. The carrier gas, the reaction gas,
and the make-up gas (if needed) are introduced at the detector
base. The base is heated and controlled by the gas chromato-
graph or allowed to track the gas chromatograph oven tem-
perature.
5.3 Reaction Tube—The nickel pyrolysis tube interfaces to
the detector base and is heated by a heating element called the
reactor which surrounds the tube. The normal operating tem-
perature is 800 to 1000°C for most applications.
5.4 Scrubber—A coiled tube, used in either the nitrogen or
sulfur mode, containing a specific scrubbing material is placed
between the exit of the pyrolysis tube and the entrance of the
conductivity cell in order to remove certain reaction products
FIG. 1 ELCD—Principal Components which may interfere in the specific mode of operation. Re-
placement of the scrubber is mandated by response to any
halogen compound.
are detected under oxidizing conditions. The effluent from the
gas chromatographic column is combined with either hydrogen 5.5 Conductivity Cell—The conductivity cell consists of a
(reducing conditions) or air (oxidizing conditions) before plastic block containing two metal electrodes that measure the
entering the heated (800 to 1000°C) nickel reaction tube. The electrolytic conductivity of the solvent. It is connected to the
compound is converted to small inorganic reaction products reactor exit by means of an inert (usually TFE-fluorocarbon)
depending upon the reaction conditions as shown in Table 1. transfer tube. It provides the conductivity signal for the specific
4.4 Table 2 shows the chemistry and modes of selective compound. Gaseous products from the reaction tube enter into
response for the detector. Depending upon the mode of the front of the cell and contact the solvent which is introduced
operation, various interfering reaction products are removed by through the side of the cell. Together, these entities pass
employing a selective gas scrubber before the product gases through the electrode area and then out through the back of the
reach the detector cell. In the nitrogen-specific mode, halogen cell.
and sulfur products are removed by reaction with a caustic
5.6 Solvent—The solvent is selected to provide the desired
scrubber. In the sulfur-specific mode, halogen products are
sensitivity and selectivity for each mode of operation. The
removed by a silver thread (or wire) scrubber. No scrubber is
solvent must be deionized, having a low conductivity, neutral
required for halogen mode operation.
pH, and must be able to dissolve the appropriate reaction
4.5 The reaction products pass to the conductivity cell
products. The increase in conductivity of the solvent due to the
where they are combined with the solvent. The following
presence of the reaction products results in a peak response
solvents are typically used for normal operation in each
corresponding to the original analyte. The solvent level in the
indicated mode. Other solvents may be used to provide
reservoir should be maintained weekly and the solvent com-
changes in selectivity and sensitivity (see 6.7):
pletely replaced every three months using highpurity solvents
Model Solvent for best results.
5.7 Solvent Delivery System—The system consists of a
Halogen 1-Propanol
Sulfur 100 % Methanol pump and an ion exchange resin system which works to both
Nitrogen 10 %t-Butyl Alcohol/90 % Water
deionize and neutralize the pH of the solvent. A by-pass system
is used to allow the pump to run at a normal speed while still
4.6 The increase in electrical conductivity of the solvent as
delivering the low solvent flow rates (30 to 100 μL/min)
a result of the introduction of the reaction products is measured
required by the detector. For operation in the nitrogen mode
by the sensing electrodes in the conductivity cell. The solvent
special solvent delivery systems may be required to ensure the
passes through the cell after being deionized through an ion
pH of the water-based solvent remains neutral. Refer to specific
exchange resin bed located between the conductivity cell and
instructions provided by the manufacturer of the respective
solvent reservoir. In most instruments the solvent is recycled
detector you are employing on your gas chromatograph. It is
by taking the solvent from the cell back into the solvent
important to note that each mode will require specific resins
reservoir.
which will require periodic replacement and attention given to
5. Detector Construction
expiration dates for their useful life-time. Resins should be
mixed thoroughly before adding or replacing as the anion/
5.1 There is some variation in the method of construction of
cation mixture used by most manufacturers will separate unless
this detector. In general, the geometry and construction of the
a prepacked resin cartridge is used.
conductivity cell is the single distinguishing component be-
tween detector designs. It is not considered pertinent to review 5.8 Module—All operational functions, except for detector
E 1698
FIG. 2 GC/ELCD System Overview
TABLE 1 Pyrolysis Reaction Products Formed Under Oxidizing
detrimental to the performance of the detector in all modes, and
or Reducing Conditions
therefore cannot be used as a carrier of makeup gas nor can it
Oxidizing Element Reducing
be tolerated as a low level contaminant. No attempt will be
CO CCH
made here to guide the selection of optimum conditions, except
2 4
HOH H
2 2
to state that experience has shown that gases of the highest
NO/N NNH
2 3
available purity result in far fewer detector problems and
HX, HOX X HX
O OH O difficulties. Poor quality, hydrogen has been found to be the
2 2
SO /SO SH S
2 3 2
cause of noise, low response, wandering baseline, and peak
tailing when operating in the halogen or nitrogen modes.
Similarly, the highest grade of air works best for the sulfur
base temperature, are controlled from the module. On some
mode.
systems, vent time can be controlled from the gas chromato-
6.3 Hardware—High-purity gases require ultra-clean regu-
graph as an external event.
lators, valves, and tubing. Use of clean regualtors, employing
5.9 Vent Valve—When opened, the vent valve provides a
stainless steel valves, diaphragms, and tubing have been found
way of preventing unwanted column effluents from entering
to result in far fewer detector problems and difficulties.
the reaction tube. These effluents may include substances such
6.4 Columns—All columns, whether packed or capillary,
as the sample injection solvent and column bleed which can
should be fully conditioned according to supplier’s specifica-
cause fouling or poisoning of the nickel reaction tube’s
tions prior to connecting to the detector. Certain liquid phases
catalytic surface. The valve is otherwise kept closed to allow
that are not compatible with the mode of operation should be
the compounds of interest to pass into the reaction tube so that
avoided. Use of silanes (such as those used in deactivation of
they may be detected. The valve interfaces with the detector
glass liners and columns) should be avoided since they have
base by means of a vent tube connected at the column exit in
been shown to poison the reactor tube.
the base. It is important that the gas flow from the vent (if used)
6.5 Reactor Temperature—The target reactor temperature is
be measured daily to ensure reproducible results and retention
800 to 900°C. However, other reactor temperatures may be
times.
found to provide better results with certain compound types.
Some typical reactor temperatures are given as follows:
6. Equipment Preparation
6.5.1 800 to 900°C for most halogen-mode applications,
6.1 The detector will be evaluated as part of a gas chro-
6.5.2 850 to 925°C for most nitrogen-mode applications,
matograph using injections of gases or liquid samples which
6.5.3 950 to 1000°C for polychlorinated biphenyls (PCBs),
have a range of component concentrations.
and
6.2 Gases—All gases passing through the reactor should be
6.5.4 900 to 950°C for sulfur compounds, such as sulfides.
ultra-high purity (99.999 %) grade. Helium or hydrogen can be
used as the GC column carrier gas. Nitrogen is extremely 6.6 Reaction Gas Flow Rate—Reaction gas flow rates fall
E 1698
TABLE 2 Reaction Products Produced in the ELCD Using a Nickel Reaction Tube
Compound Main Reaction Products Comments
Reductive Conditions:
Halogen compounds HX HX can be removed by N-mode scrubber and is selectively detected in X-mode.
Sulfur compounds HSH S can be removed by N-mode scrubber and is poorly ionized in the X-mode.
2 2
Nitrogen compounds NH NH is poorly ionized in the X-mode and selectively detected in N-mode.
3 3
Alkanes CH , Lower Alkanes Products are not ionized in any mode.
Oxygen compounds HOH O gives little response in X-mode and N-mode.
2 2
Oxidative Conditions:
Halogen compounds HX, HOX HX can be removed by S-mode scrubber.
Sulfur compounds SO SO is selectively detected in S-mode.
2 2
Nitrogen compounds N and certain nitrogen oxides at No or little response.
elevated temperatures
Alkanes CO ,HOCO is poorly ionized in S-mode. H O gives little or no response.
2 2 2 2
wit
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.