ASTM D2384-83(2000)e1
(Test Method)Standard Test Methods for Traces of Volatile Chlorides in Butane-Butene Mixtures
Standard Test Methods for Traces of Volatile Chlorides in Butane-Butene Mixtures
SCOPE
1.1 These test methods cover the determination of the total volatile organic chlorides in concentrations from 10 to 100 ppm in butane-butene mixtures. The amperometric finish is not directly applicable in the presence of other substances that combine with silver ion or oxidize chloride ion in dilute acid solution. Bromides, sulfides, ammonia, tobacco smoke, and more than 25 µg of hydrogen peroxide in the test solution interfere in the spectrophotometric procedure.
1.2 Dissolved sodium chloride is not quantitatively determined using these test methods.
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use. Specific precautionary statements are given in Sections 5, 8, 11, 14, 19, and Annex A1.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn. Contact ASTM
International (www.astm.org) for the latest information.
An American National Standard
e1
Designation:D2384–83 (Reapproved 2000)
Standard Test Methods for
Traces of Volatile Chlorides in Butane-Butene Mixtures
This standard is issued under the fixed designation D2384; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
e NOTE—Warning notes were placed in the text editorially in November 2000.
Although an oxy-hydrogen burner does not lend itself to multiple testing,
1. Scope
itaffordsmuchmorerapidanalysisforasinglesamplethandoesthelamp
1.1 These test methods cover the determination of the total
combustion.
volatile organic chlorides in concentrations from 10 to 100
3.1.1 Lamp Combustion—Thesampleisburnedinanatmo-
ppminbutane-butenemixtures.Theamperometricfinishisnot
sphere of carbon dioxide and oxygen or in purified air; the
directly applicable in the presence of other substances that
halogen-containingcombustionproductsareabsorbedindilute
combine with silver ion or oxidize chloride ion in dilute acid
sodium carbonate solution.
solution. Bromides, sulfides, ammonia, tobacco smoke, and
3.1.2 Oxy-Hydrogen Combustion—The sample is burned in
more than 25 µg of hydrogen peroxide in the test solution
anoxy-hydrogenatomizerburner,andthecombustionproducts
interfere in the spectrophotometric procedure.
are absorbed in a dilute solution of sodium carbonate.
1.2 Dissolved sodium chloride is not quantitatively deter-
3.2 Finishes—Either the amperometric titration or spectro-
mined using these test methods.
photometric finish may be used for the chloride ion determi-
1.3 This standard does not purport to address all of the
nation.
safety concerns, if any, associated with its use. It is the
3.2.1 Amperometric Titration—The chloride ion in aqueous
responsibility of the user of this standard to establish appro-
solution is titrated amperometrically with standard silver ni-
priate safety and health practices and determine the applica-
trate solution, using a saturated calomel electrode as reference
bility of regulatory limitations prior to use. Specific precau-
electrode. The diffusion currents are plotted against the corre-
tionary statements are given in Sections 5, 8, 11, 14, 19, and
sponding volumes of silver nitrate solution used; the end point
Annex A1.
is taken as the intersection of the two straight-line portions of
2. Referenced Documents the curve.
3.2.2 Spectrophotometric Finish—Chloride ion in the ab-
2.1 ASTM Standards:
2 sorber solution is determined by reaction with mercuric thio-
D329 Specification for Acetone
cyanate to release thiocyanate, which forms a reddish orange
D1266 Test Method for Sulfur in Petroleum Products
+++
3 complex with Fe . The intensity of the color is measured at
(Lamp Method)
460 nm with a spectrophotometer or filter photometer.
3. Summary of Test Methods
4. Significance and Use
3.1 Combination Methods—Either the lamp or oxy-
4.1 These test methods are used to determine trace amounts
hydrogen method may be used for combustion.
of volatile chlorides in butane-butene mixtures. Such informa-
NOTE 1—Lamp combustion is readily applicable to multiple testing.
tionisvaluableincaseswherechlorideisdeleteriousintheuse
of this product; also, chloride contributes to corrosion prob-
lems in processing units in instances where further processing
These test methods are under the jurisdiction of ASTM Committee D02 on
of this material is involved.
Petroleum Products and Lubricants and are the direct responsibility of Subcommit-
tee D02.D0.04 on C Hydrocarbons.
Current edition approved Oct. 28, 1983. Published January 1984. Originally
published as D2384 – 65 T. Last previous edition D2384– 68 (1978).
Annual Book of ASTM Standards, Vol 06.04.
Annual Book of ASTM Standards, Vol 05.01.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn. Contact ASTM
International (www.astm.org) for the latest information.
D2384
5. Purity of Reagents 6.3 Obtain a liquid sample from the purged sample line,
filling the upright cylinder through the bottom needle valve,
5.1 Purity of Reagents—Reagent grade chemicals shall be
keepingthetopvalveclosed.Donotpurgethesamplecylinder.
used in all tests. Unless otherwise indicated, it is intended that
all reagents shall conform to the specifications of the Commit-
LAMP COMBUSTION METHOD
tee onAnalytical Reagents of theAmerican Chemical Society,
where such specifications are available. Other grades may be
7. Apparatus
used, provided it is first ascertained that the reagent is of
7.1 ASTM Lamp Assembly—Use the apparatus specified in
sufficiently high purity to permit its use without lessening the
Test Method D1266, including the liquified petroleum gas
accuracy of the determination.
burner assembly.
5.2 References to water shall be understood to mean
chloride-free distilled or deionized water.
8. Reagents
5.3 Warning—In view of the common occurrence of chlo-
8.1 Use the necessary reagents and materials specified in
ride in reagents and laboratory air, special care must be taken
Test Method D1266, in addition to the absorber solution as
during preparation and storage of reagents to avoid contami-
described in 8.3.
nation. They should be isolated from other reagents and used
solely for these methods. A blank determination must be 8.2 Hydrogen (Warning—Extremely flammable (liquified)
gas under pressure. See Annex A1.1.)
performedeachtimeareagentischangedtoensurethatitisnot
contaminated with chloride. 8.3 Sodium Carbonate Absorbent (2 g/L)— Dissolve 2.0 g
of anhydrous sodium carbonate (Na CO ) in water and dilute
It is also imperative that all glassware used in this determi-
2 3
nation be cleaned thoroughly and rinsed four times with to a litre with water.
chloride-free distilled or deionized water. Utmost caution must
9. Procedure
be taken during the analysis to prevent contamination from
chlorides.
9.1 Prepare the combustion apparatus as described in Sec-
tion7ofTestMethodD1266using35mLofNa CO solution
2 3
6. Sampling
to charge the absorber.
9.2 Weigh the vessel containing the sample to the nearest
6.1 Steam and dry a 10 to 25-mL corrosion-resistant metal
sample cylinder having a 450-psi (3100 kPa) working pressure 0.1 g. Support the sample vessel in an upright position so that
the sample is burned from the gaseous phase. Connect the
and equipped with a needle valve outlet at each end.
6.2 Pressure the prepared cylinder with dry hydrogen to 20 sample vessel to the auxiliary corrosion-resistant regulating
valve by means of corrosion-resistant metal tubing (Fig. 1)
psig (137.5 kPa gage) to afford a gas cushion preventing
rupture due to liquid expansion on increase of temperature. (Note 2). Connect the bottom valve of the sample vessel to the
regulated hydrogen supply. By means of short lengths of
chloride-free rubber tubing, connect the auxiliary valve outlet
to the side inlet of the gas burner and the lower inlet of the gas
burner (Test Method D1266, Appendix A3, Fig. 5) to the
Reagent Chemicals, American Chemical Society Specifications, American
Chemical Society, Washington, DC. For suggestions on the testing of reagents not
burner manifold.
listed by the American Chemical Society, see Analar Standards for Laboratory
Chemicals, BDH Ltd., Poole, Dorset, U.K., and the United States Pharmacopeia NOTE 2—For steady burning, it may be necessary to surround the
and National Formulary, U.S. Pharmacopeial Convention, Inc. (USPC), Rockville,
auxiliary valve with a heat-exchanger system. A convenient means is
MD.
FIG. 1 Diagrammatic Sketch of Butane-Butene Heat Exchange System
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn. Contact ASTM
International (www.astm.org) for the latest information.
D2384
winding insulated heating wire, having a resistance of 40 to 60 V, around
theauxiliaryvalveandconnectingittoasuitablerheostat.Anothermeans
is to place the regulating valve in a suitable metal beaker and cover the
valve body with water maintained at 60 to 80°C.
9.3 Open the valve on the sample vessel; then open the
auxiliary valve to allow a small stream of vapor to escape.
Quickly light the burner. Adjust the flow of CO -O mixture
2 2
and the sample so that the flame is approximately 35 mm high
and clear blue in color; this color is reached just beyond the
point at which a yellow color shows at the tip of the flame.
Insert the burner into the chimney and readjust the flame if
necessary. When the sample has burned almost to completion,
openthevalveonthebottomofthesamplevesselandflushthe
residual sample from the cylinder chamber by passing hydro-
gen through the bottom valve for several minutes.
9.4 When all of the residual material has been flushed from
thesamplevessel,turnoffthehydrogenandclosethevalveson
the sample vessel. Disconnect the hydrogen flushing line and
the line to the heated auxiliary valve and weigh the sample
vessel to the nearest 0.1 g. Draw the combustion atmosphere
through one absorber of a set to serve as a blank on the purity
of this atmosphere. Rinse the chimneys and spray traps with
1—Atomizer-burner 4—Three-way stopcock
water and add the rinsings to the absorbers.
2—Sample tube 5—Absorber
9.5 Proceed in accordance with either Section 16 or 21.
3—Combustion chamber 6—Spray trap
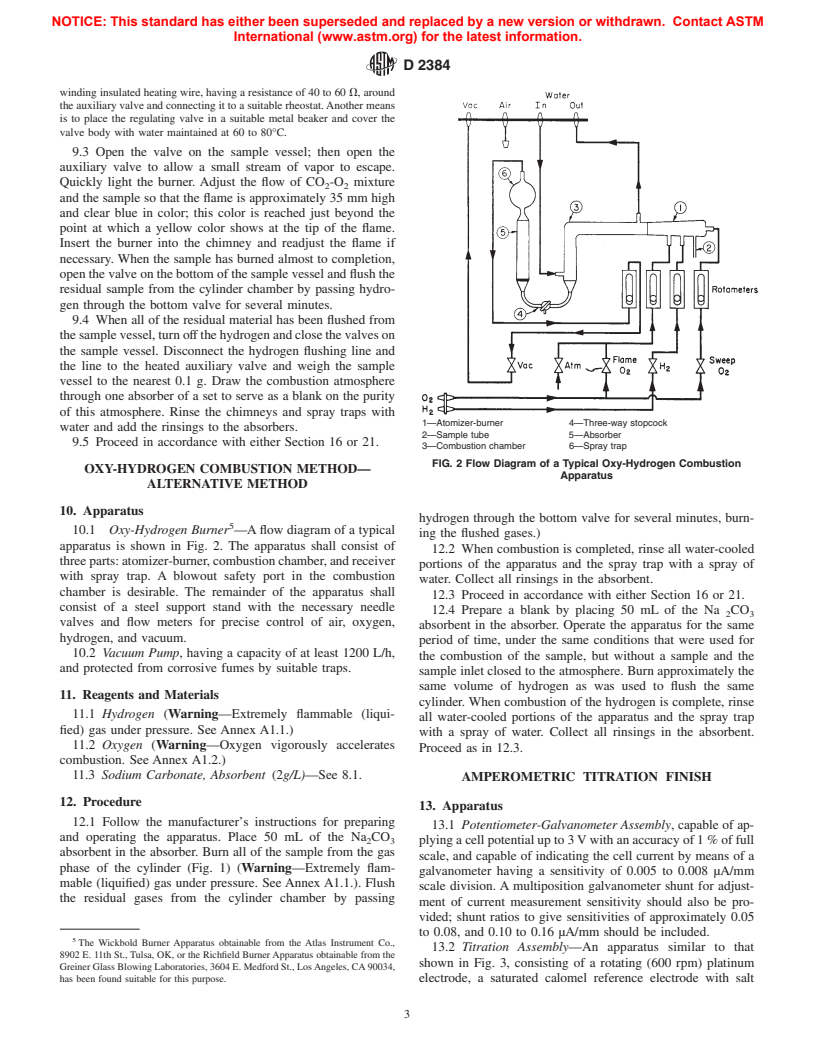
FIG. 2 Flow Diagram of a Typical Oxy-Hydrogen Combustion
OXY-HYDROGEN COMBUSTION METHOD—
Apparatus
ALTERNATIVE METHOD
10. Apparatus
hydrogen through the bottom valve for several minutes, burn-
10.1 Oxy-Hydrogen Burner —Aflow diagram of a typical
ing the flushed gases.)
apparatus is shown in Fig. 2. The apparatus shall consist of
12.2 When combustion is completed, rinse all water-cooled
threeparts:atomizer-burner,combustionchamber,andreceiver
portions of the apparatus and the spray trap with a spray of
with spray trap. A blowout safety port in the combustion
water. Collect all rinsings in the absorbent.
chamber is desirable. The remainder of the apparatus shall
12.3 Proceed in accordance with either Section 16 or 21.
consist of a steel support stand with the necessary needle
12.4 Prepare a blank by placing 50 mL of the Na CO
2 3
valves and flow meters for precise control of air, oxygen,
absorbent in the absorber. Operate the apparatus for the same
hydrogen, and vacuum.
period of time, under the same conditions that were used for
10.2 Vacuum Pump, having a capacity of at least 1200 L/h,
the combustion of the sample, but without a sample and the
and protected from corrosive fumes by suitable traps.
sample inlet closed to the atmosphere. Burn approximately the
same volume of hydrogen as was used to flush the same
11. Reagents and Materials
cylinder. When combustion of the hydrogen is complete, rinse
11.1 Hydrogen (Warning—Extremely flammable (liqui-
all water-cooled portions of the apparatus and the spray trap
fied) gas under pressure. See Annex A1.1.)
with a spray of water. Collect all rinsings in the absorbent.
11.2 Oxygen (Warning—Oxygen vigorously accelerates
Proceed as in 12.3.
combustion. See Annex A1.2.)
11.3 Sodium Carbonate, Absorbent (2g/L)—See 8.1.
AMPEROMETRIC TITRATION FINISH
12. Procedure
13. Apparatus
12.1 Follow the manufacturer’s instructions for preparing
13.1 Potentiometer-Galvanometer Assembly, capable of ap-
and operating the apparatus. Place 50 mL of the Na CO
2 3
plyingacellpotentialupto3Vwithanaccuracyof1%offull
absorbent in the absorber. Burn all of the sample from the gas
scale, and capable of indicating the cell current by means of a
phase of the cylinder (Fig. 1) (Warning—Extremely flam-
galvanometer having a sensitivity of 0.005 to 0.008 µA/mm
mable (liquified) gas under pressure. See Annex A1.1.). Flush
scale division. A multiposition galvanometer shunt for adjust-
the residual gases from the cylinder chamber by passing
ment of current measurement sensitivity should also be pro-
vided; shunt ratios to give sensitivities of approximately 0.05
to 0.08, and 0.10 to 0.16 µA/mm should be included.
The Wickbold Burner Apparatus obtainable from the Atlas Instrument Co.,
13.2 Titration Assembly—An apparatus similar to that
8902 E. 11th St., Tulsa, OK, or the Richfield BurnerApparatus obtainable from the
shown in Fig. 3, consisting of a rotating (600 rpm) platinum
GreinerGlassBlowingLaboratories,3604E.MedfordSt.,LosAngeles,CA90034,
has been found suitable for this purpose. electrode, a saturated calomel reference electrode with salt
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn. Contact ASTM
International (www.astm.org) for the latest information.
D2384
FIG. 3 Schematic Assembly of the Amperometric Titration Cell
NOTE 3—Remove possible objectionable amounts of chloride by pass-
bridge, a means of blanketing the solution with nitrogen, and a
ing the solution through an ion-exchange resin in the hydroxyl form.
holder for a 10-mL buret.
Neutralizetheresultingalkalinesolutiontothebromthymolblueendpoint
13.3 Calomel Electrode, constructed as shown in Fig. 3.
by titration with HNO (3+97).
13.4 Platinum Electrode, rotating-hook type. A suitable
−
14.4 Chloride, Standard Solution (10 µg Cl /mL)—Dilute a
electrode may be constructed as follows: Seal a platinum wire
suitable volume of 1+10 assayed hydrochloric acid to obtain
0.03to0.05in.(0.76to1.3mm)indiameterand0.75to1.0in.
−
a solution containing 10 µg Cl /mL.
(19.1to25.4mm)longintotheendofa6-mmoutsidediameter
14.5 Gelatin Solution (10 g/L)—Dissolve1gof gelatin in
soft glass tube that has been shaped into a stirrer blade. Bend
100 mL of hot water and add 1 mL of chloroform as a
theextendingplatinumwireupwardsatitsmidpointtoforman
preservative. Discard the solution when it is 1 week old.
angleof90°.Placeafewdropsofmercuryintheglasstubeand
14.6 Hydrogen Peroxide Solution—Prepare by diluting 1
make electrical contact between the mercury and the connec-
volume of concentrated hydrogen peroxide solution (H O ,
tion on the amperometric titrator with a piece of copper wire
2 2
30%) with 4 volumes of water. Store in a dark-colored
(insulate the exposed wire to prevent shorting).
glass-stoppered bottle.
13.5 Buret—A 10-mL semi-micro buret, with the tip con-
14.7 Mercury-Calomel Mixture—Prepare a mixture of mer-
structedsoastobeabletodipbelowthesurfaceofthesolution
cury and calomel (mercurous chloride, Hg Cl ) by vigorously
being titrated.
2 2
shaking 10 g of calomel with 50 g of mercury, in a glass-
14. R
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.