ASTM D4765-03(2008)
(Test Method)Standard Test Method for Fluorides in Workplace Atmospheres
Standard Test Method for Fluorides in Workplace Atmospheres
SIGNIFICANCE AND USE
The capability of this test method to collect and quantitate both particulate and gaseous fluorides over the ranges normally encountered in industrial atmospheres makes it applicable for industrial hygiene evaluation and control purposes. The recommended range of this test method is from 0.005 to 5 mg F−/m3 air.
SCOPE
1.1 This test method covers the simultaneous collection and separate measurements of gaseous and particulate fluoride found in certain industrial workplaces. The gaseous inorganic fluorides collected are reported in terms of fluoride; the procedure is not applicable to the collection or analysis of other fluoride-bearing gases (for example, fluorocarbon or fluorosulfur compounds). This test method covers sample collection, preparation, and fluoride measurement.
1.2 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Buy Standard
Standards Content (Sample)
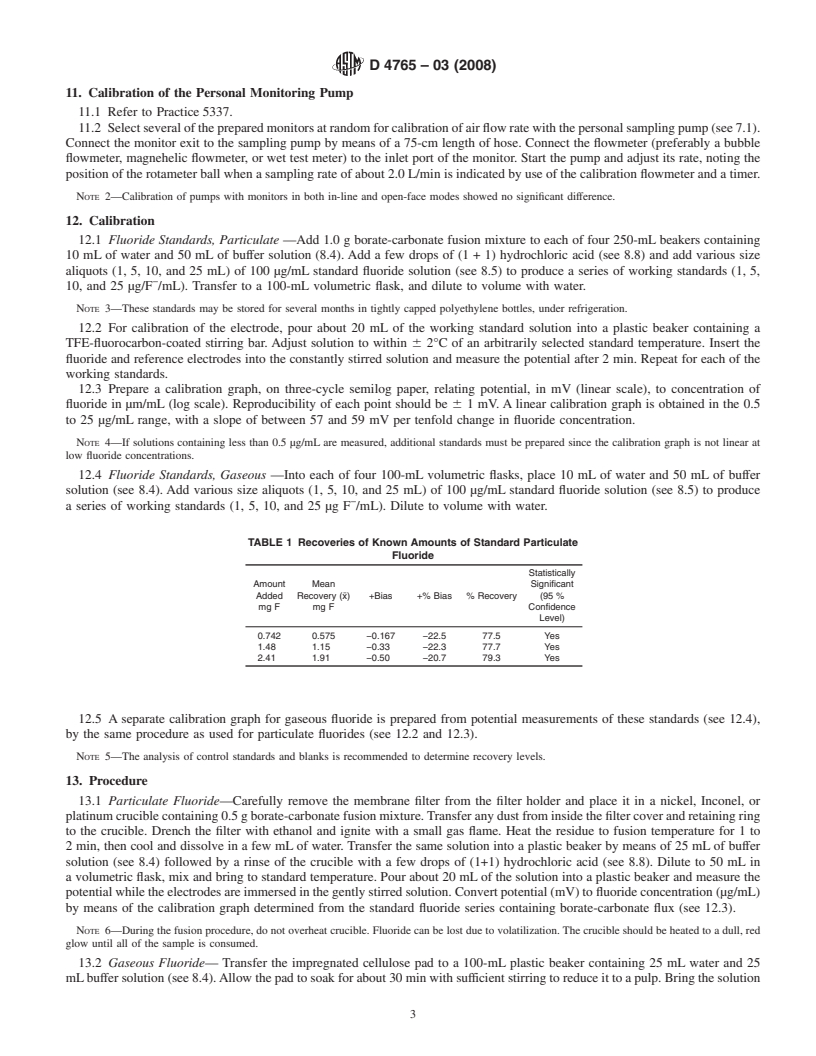
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: D4765 − 03(Reapproved 2008)
Standard Test Method for
Fluorides in Workplace Atmospheres
This standard is issued under the fixed designation D4765; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope from the sample of air, is absorbed by an alkali-impregnated
cellulose pad placed immediately behind the membrane filter.
1.1 This test method covers the simultaneous collection and
The membrane filter and collected solids are made alkaline,
separate measurements of gaseous and particulate fluoride
ashed, and the residue fused with additional alkali. Finally, the
found in certain industrial workplaces. The gaseous inorganic
fluoride is determined in a solution of the melt by use of a
fluorides collected are reported in terms of fluoride; the
fluorideion-selectiveelectrode.Gaseousfluorideisdetermined
procedureisnotapplicabletothecollectionoranalysisofother
inanaqueousextractofthecellulosepad,alsobymeansofthe
fluoride-bearing gases (for example, fluorocarbon or fluorosul-
fluoride ion-selective electrode.
fur compounds). This test method covers sample collection,
preparation, and fluoride measurement.
5. Significance and Use
1.2 This standard does not purport to address all of the
5.1 The capability of this test method to collect and quan-
safety concerns, if any, associated with its use. It is the
titate both particulate and gaseous fluorides over the ranges
responsibility of the user of this standard to establish appro-
normally encountered in industrial atmospheres makes it ap-
priate safety and health practices and determine the applica-
plicableforindustrialhygieneevaluationandcontrolpurposes.
bility of regulatory limitations prior to use.
The recommended range of this test method is from 0.005 to 5
− 3
mg F /m air.
2. Referenced Documents
2.1 ASTM Standards:
6. Interferences
D1193Specification for Reagent Water
6.1 Because an ion-selective electrode responds to ionic
D1356Terminology Relating to Sampling and Analysis of
activity, insoluble and complex forms of fluoride must be
Atmospheres
released by appropriate combinations of fusion, adjustment of
D1357Practice for Planning the Sampling of the Ambient
pH, and addition of complexing agents.
Atmosphere
6.2 Acidity (pH) and ionic strengths of fluoride standard
D5337Practice for Flow RateAdjustment of Personal Sam-
solutions must be matched to those of samples.
pling Pumps
6.3 Temperature of sample and standard solutions must be
3. Terminology
controlled within 62°C.
3.1 Definitions—For definitions of terms used in this test
method, refer to Terminology D1356. 7. Apparatus
7.1 Personal Sampling Pump, Equipped with a flow-
4. Summary of Test Method
monitoring device (rotameter, critical orifice) or a constant-
4.1 Particulate material from a measured volume of air is
flow device capable of drawing 2 L/min of air through the
collected by means of a membrane filter. Gaseous fluoride,
0.8-µm membrane filter and pad for a period of 8 h.
7.2 Filter Holder—Plasticholdersofthepreloadedpersonal
This test method is under the jurisdiction of ASTM Committee D22 on Air
monitor type, that accept filters of 37-mm diameter, are
Qualityand is the direct responsibility of Subcommittee D22.04 on Workplace Air
preferred. The holder is to be numbered for identification.
Quality.
Current edition approved April 1, 2008. Published July 2008. Originally
7.3 MembraneFilter,ofmixed-celluloseesters,0.8-µmpore
approved in 1988. Last previous edition approved in 2003 as D4765–03. DOI:
size, and of diameter to fit the filter holder (see 7.2).
10.1520/D4765-03R08.
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
7.4 Cellulose Pad, of size to fit the filter holder (see 7.2).
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
The pad is commercially available as a plain, unpregnated pad
Standards volume information, refer to the standard’s Document Summary page on
the ASTM website. or as an alkali-impregnated pad.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
D4765 − 03 (2008)
7.5 Crucibles, 20-mL, nickel, Inconel, or platinum. worker’s collar and remove the plug for closed-face sampling.
Air is drawn through the filter at the calibrated rate of
7.6 Fluoride Ion-Selective Electrode.
approximately 2.0 L/min and maintained at that rate by
7.7 Reference Electrode,calomeltype,preferablycombined
occasional checking and adjustment. On termination of
with the fluoride ion-selective electrode.
sampling,notethedurationofsampling,resealthemonitorand
7.8 Electrometer or Expanded Scale pH Meter, with a return the monitor to the laboratory. Filter a minimum air
sample of 250 L.
millivolt scale for measurement of potentials.
7.9 Magnetic Stirrer. 9.3 Totalparticulateloadingmaybedetermined,ifrequired,
by pre- and post-weighing of the membrane filter.
7.10 Plastic Beakers, 50 and 100-mL capacities.
7.11 Beakers, 250-mL capacity.
10. Preparation of Monitors
7.12 Volumetric Flasks, 100-mL capacity.
10.1 Disassemble the personal monitor (see 7.2), removing
the membrane filter and cellulose pads. Moisten the pad with a
8. Reagents
measuredvolumeofalkalinefixativesolution(see8.3);0.8mL
8.1 Purity of Reagents—Reagent grade chemicals shall be
is required for a pad of 37-mm diameter. Dry the pad at 105°C
used in all tests. Unless otherwise indicated, it is intended that for 30 to 45 min.
all reagents shall conform to the specifications of the Commit-
NOTE 1—Preparation of alkali-impregnated pads must be carried out in
tee onAnalytical Reagents of theAmerican Chemical Society,
a low-fluoride environment with minimum exposure.
where such specifications are available.
10.2 Reassemble the filter monitor, inserting an impreg-
8.2 Purity of Water—Unless otherwise indicated, references
nated pad and membrane filter, and closing with the filter
to water shall be understood to mean Type I Reagent Water
retaining ring and front cover. Seal the assembly against air
conforming to Specification D1193.
leakage by a wrap of masking tape or cellulose shrink bands,
coveringthecrevicebetweentheretainingringandbackcover.
8.3 Alkaline Fixative Solution—Dissolve 25 g of sodium
Close the inlet and outlet openings of the monitor with plastic
carbonate(Na CO )inwater,add20mLglycerol,anddiluteto
2 3
plugs.
1L.
8.4 Buffer Solution (ALCOA)—Dissolve 60 g of citric acid
11. Calibration of the Personal Monitoring Pump
monohydrate (C H O ·H O), 210 g of sodium citrate
2 8 7 2
11.1 Refer to Practice D5337.
(Na C H O ·2H O) and 53.5 g of ammonium chloride
3 6 5 7 2
(NH Cl)in500mLwater.Add67mLofammoniumhydroxide
4 11.2 Select several of the prepared monitors at random for
(NH OH) (sp gr=0.90) and dilute to 1 L with water.
4 calibration of air flow rate with the personal sampling pump
(see 7.1). Connect the monitor exit to the sampling pump by
8.5 Fluoride Solution, Standard (100 µg/mL)—Dissolve
means of a 75-cm length of hose. Connect the flowmeter
0.2211gsodiumfluoride(NaF,driedat105°Cfor2h)inwater
(preferably a bubble flowmeter, magnehelic flowmeter, or wet
and dilute to volume in a 1-L volumetric flask.
test meter) to the inlet port of the monitor. Start the pump and
8.6 Ethanol, Denatured—Formula 30 denatured alcohol is
adjust its rate, noting the position of the rotameter ball when a
satisfactory.
sampling rate of about 2.0 L/min is indicated by use of the
8.7 Borate-Carbonate Fusion Mixture—Intimately mix a
calibration flowmeter and a timer.
1+2 (w/w) combination of sodium tetraborate (Na B O ) and
2 4 7
NOTE 2—Calibration of pumps with monitors in both in-line and
sodium carbonate (Na CO ).
2 3
open-face modes showed no significant difference.
8.8 Hydrochloric Acid (1+1)—Mix one part hydrochloric
12. Calibration
acid to one part distilled water as a solution.
12.1 Fluoride Standards, Particulate—Add 1.0 g borate-
9. Sampling
carbonate fusion mixture to each of four 250-mL beakers
9.1 For general information on sampling, refer to Practice
containing 10 mLof water and 50 mLof buffer solution (8.4).
D1357.
Add a fewdrops of (1 +1) hydrochloric acid (see8.8) and add
various size aliquots (1, 5, 10, and 25 mL) of 100 µg/mL
9.2 Equip the worker whose exposure is to be evaluated
standard fluoride solution (see 8.5) to produce a series of
with a personal monitor connected by a 75-cm length of hose
−
working standards (1, 5, 10, and 25 µg/F /mL). Transfer to a
to a belt-supported sampling pump. Attach the monitor to the
100-mL volumetric flask, and dilute to volume with water.
NOTE 3—These
...
This document is not anASTM standard and is intended only to provide the user of anASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
Designation:D4765–98 Designation:D4765–03 (Reapproved 2008)
Standard Test Method for
Fluorides in Workplace Atmospheres
This standard is issued under the fixed designation D4765; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope
1.1 This test method covers the simultaneous collection and separate measurements of gaseous and particulate fluoride found
in certain industrial workplaces. The gaseous inorganic fluorides collected are reported in terms of fluoride; the procedure is not
applicabletothecollectionoranalysisofotherfluoride-bearinggases(forexample,fluorocarbonorfluorosulfurcompounds).This
test method covers sample collection, preparation, and fluoride measurement.
1.2 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility
of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory
limitations prior to use.
2. Referenced Documents
2.1 ASTM Standards:
D1193 Specification for Reagent Water
D1356 Terminology Relating to Sampling and Analysis of Atmospheres
D1357 Practice for Planning the Sampling of the Ambient Atmosphere
D5337 Practice for Flow Rate for Calibration of Personal Sampling Pumps
3. Terminology
3.1 Definitions— For definitions of terms used in this test method, refer to Terminology D1356.
4. Summary of Test Method
4.1 Particulate material from a measured volume of air is collected by means of a membrane filter. Gaseous fluoride, from the
sample of air, is absorbed by an alkali-impregnated cellulose pad placed immediately behind the membrane filter. The membrane
filter and collected solids are made alkaline, ashed, and the residue fused with additional alkali. Finally, the fluoride is determined
in a solution of the melt by use of a fluoride ion-selective electrode. Gaseous fluoride is determined in an aqueous extract of the
cellulose pad, also by means of the fluoride ion-selective electrode.
5. Significance and Use
5.1 The capability of this test method to collect and quantitate both particulate and gaseous fluorides over the ranges normally
encountered in industrial atmospheres makes it applicable for industrial hygiene evaluation and control purposes. The
− 3
recommended range of this test method is from 0.005 to 5 mg F /m air.
6. Interferences
6.1 Because an ion-selective electrode responds to ionic activity, insoluble and complex forms of fluoride must be released by
appropriate combinations of fusion, adjustment of pH, and addition of complexing agents.
6.2 Acidity (pH) and ionic strengths of fluoride standard solutions must be matched to those of samples.
6.3 Temperature of sample and standard solutions must be controlled within 62°C.
This test method is under the jurisdiction ofASTM Committee D-22 on Sampling andAnalysis ofAtmospheres and is the direct responsibility of Subcommittee D22.04
on Workplace Atmospheres.
Current edition approved May 10, 1998. Published July 1998. Originally published as D4765–88. Last previous edition D4765–93.
This test method is under the jurisdiction ofASTM Committee D22 onAir Quality and is the direct responsibility of Subcommittee D22.04 on WorkplaceAir Quality
.
Current edition approved April 1, 2008. Published July 2008. Originally approved in 1988. Last previous edition approved in 2003 as D4765–03.
ForreferencedASTMstandards,visittheASTMwebsite,www.astm.org,orcontactASTMCustomerServiceatservice@astm.org.For Annual Book of ASTM Standards
, Vol 11.01.volume information, refer to the standard’s Document Summary page on the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
D4765–03 (2008)
7. Apparatus
7.1 Personal Sampling Pump, Equipped with a flow-monitoring device (rotameter, critical orifice) or a constant-flow device
capable of drawing 2 L/min of air through the 0.8-µm membrane filter and pad for a period of 8 h.
7.2 Filter Holder—Plasticholdersofthepreloadedpersonalmonitortype,thatacceptfiltersof37-mmdiameter,arepreferred.
The holder is to be numbered for identification.
7.3 Membrane Filter, of mixed-cellulose esters, 0.8-µm pore size, and of diameter to fit the filter holder (see 7.2).
7.4 Cellulose Pad, of size to fit the filter holder (see 7.2). The pad is commercially available as a plain, unpregnated pad or as
an alkali-impregnated pad.
7.5 Crucibles, 20-mL, nickel, Inconel, or platinum.
7.6 Fluoride Ion-Selective Electrode .
7.7 Reference Electrode, calomel type, preferably combined with the fluoride ion-selective electrode.
7.8 Electrometer or Expanded Scale pH Meter, with a millivolt scale for measurement of potentials.
7.9 Magnetic Stirrer.
7.10 Plastic Beakers, 50 and 100-mL capacities.
7.11 Beakers, 250-mL capacity.
7.12 Volumetric Flasks, 100-mL capacity.
8. Reagents
8.1 Purity of Reagents—Reagent grade chemicals shall be used in all tests. Unless otherwise indicated, it is intended that all
reagents shall conform to the specifications of the Committee on Analytical Reagents of the American Chemical Society, where
such specifications are available.
8.2 Purity of Water— Unless otherwise indicated, references to water shall be understood to mean Type I Reagent Water
conforming to Specification D1193.
8.3 Alkaline Fixative Solution —Dissolve 25 g of sodium carbonate (Na CO ) in water, add 20 mLglycerol, and dilute to 1 L.
2 3
8.4 Buffer Solution (ALCOA)—Dissolve 60 g of citric acid monohydrate (C H O ·H O), 210 g of sodium citrate
2 8 7 2
(NaC H O ·2H O) and 53.5 g of ammonium chloride (NH Cl) in 500 mLwater.Add 67 mLof ammonium hydroxide (NH OH)
6 5 7 2 4 4
(sp gr=0.90) and dilute to 1 L with water.
8.5 Fluoride Solution, Standard (100 µg/mL)—Dissolve 0.2211 g sodium fluoride (NaF, dried at 105°C for 2 h) in water and
dilute to volume in a 1-L volumetric flask.
8.6 Ethanol, Denatured—Formula 30 denatured alcohol is satisfactory.
8.7 Borate-Carbonate Fusion Mixture—Intimatelymixa1+2(w/w)combinationofsodiumtetraborate(Na B O )andsodium
2 4 7
carbonate (Na CO ).
2 3
8.8 Hydrochloric Acid (1+1)—Mix one part hydrochloric acid to one part distilled water as a solution.
9. Sampling
9.1 For general information on sampling, refer to Practice D1357.
9.2 Equip the worker whose exposure is to be evaluated with a personal monitor connected by a 75-cm length of hose to a
belt-supported sampling pump. Attach the monitor to the worker’s collar and remove the plug for closed-face sampling. Air is
drawn through the filter at the calibrated rate of approximately 2.0 L/min and maintained at that rate by occasional checking and
adjustment.Onterminationofsampling,notethedurationofsampling,resealthemonitorandreturnthemonitortothelaboratory.
Filter a minimum air sample of 250 L.
9.3 Total particulate loading may be determined, if required, by pre- and post-weighing of the membrane filter.
10. Preparation of Monitors
10.1 Disassemble the personal monitor (see 7.2), removing the membrane filter and cellulose pads. Moisten the pad with a
measured volume of alkaline fixative solution (see 8.3); 0.8 mL is required for a pad of 37-mm diameter. Dry the pad at 105°C
for 30 to 45 min.
NOTE 1—Preparation of alkali-impregnated pads must be carried out in a low-fluoride environment with minimum exposure.
10.2 Reassemble the filter monitor, inserting an impregnated pad and membrane filter, and closing with the filter retaining ring
and front cover. Seal the assembly against air leakage by a wrap of masking tape or cellulose shrink bands, covering the crevice
between the retaining ring and back cover. Close the inlet and outlet openings of the monitor with plastic plugs.
Annual Book of ASTM Standards, Vol 11.03.
Inconel is a trademark for a group of corrosion-resistant alloys of nickel and chromium.
Inconel is a trademark for a group of corrosion-resistant alloys of nickel and chromium.
Reagent Chemicals, American Chemical Society Specifications ,American Chemical Society, Washington, DC. For suggestions on the testing of reagents not listed by
the American Chemical Society, see Analar Standards for Laboratory Chemicals, BDH Ltd., Poole, Dorset, U.K., and the United States Pharmacopeia and National
Formulary, U.S. Pharmacopeial Convention, Inc. (USPC), Rockville, MD.
D4765–03 (2008)
11. Calibration of the Personal Monitoring Pump
11.1 Refer to Practice5337.
11.2 Selectseveralofthepreparedmonitorsatrandomforcalibrationofairflowratewiththepersonalsamplingpump(see7.1).
Connect the monitor exit to the sampling pump by means of a 75-cm length of hose. Connect the flowmeter (preferably a bubble
flowmeter, magnehelic flowmeter, or wet test meter) to the inlet port of the monitor. Start the pump and adjust its rate, noting
...









Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.