ASTM E1252-98
(Practice)Standard Practice for General Techniques for Obtaining Infrared Spectra for Qualitative Analysis
Standard Practice for General Techniques for Obtaining Infrared Spectra for Qualitative Analysis
SCOPE
1.1 This practice covers the spectral range from 4000-50 cm -1 and includes techniques that are useful for qualitative analysis of liquid-, solid-, and vapor-phase samples by infrared spectrometric techniques for which the amount of sample available for analysis is not a limiting factor.
1.2 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use. Specific precautions are given in 6.5.1.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superceded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
Designation: E 1252 – 98
Standard Practice for
General Techniques for Obtaining Infrared Spectra for
Qualitative Analysis
This standard is issued under the fixed designation E 1252; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope describes methods for the proper application of infrared
spectroscopy.
1.1 This practice covers the spectral range from 4000–50
−1
cm and includes techniques that are useful for qualitative
5. General
analysis of liquid-, solid-, and vapor-phase samples by infrared
5.1 Infrared (IR) qualitative analysis is carried out by
spectrometric techniques for which the amount of sample
functional group identification (1-3) or by the comparison of
available for analysis is not a limiting factor. These techniques
IR absorption spectra of unknown materials with those of
are often also useful for recording spectra at frequencies higher
–1
known reference materials, or both. These spectra are obtained
than 4000 cm , in the near-infrared region.
(4-8) through transmission, reflection, and other techniques,
1.2 This standard does not purport to address all of the
such as photoacoustic spectroscopy (PAS). Spectra that are to
safety concerns, if any, associated with its use. It is the
be compared should be obtained by the same technique and
responsibility of the user of this standard to establish appro-
under the same conditions. Users of published reference
priate safety and health practices and determine the applica-
spectra (9-16) should be aware that not all of these spectra are
bility of regulatory limitations prior to use. Specific precau-
fully validated.
tions are given in 6.5.1.
5.1.1 Instrumentation and accessories for infrared qualita-
2. Referenced Documents tive analysis are commercially available. The manufacturer’s
manual should be followed to ensure optimum performance
2.1 ASTM Standards:
and safety.
E 131 Terminology Relating to Molecular Spectroscopy
5.2 Transmission spectra are obtained by placing a thin
E 168 Practices for General Techniques of Infrared Quanti-
uniform layer of the sample perpendicular to the infrared
tative Analysis
radiation path (see 9.5.1 for exception in order to eliminate
E 334 Practices for General Techniques of Infrared Mi-
interference fringes for thin films). The sample thickness must
croanalysis
be adequate to cause a decrease in the radiant power reaching
E 573 Practices for Internal Reflection Spectroscopy
the detector at the absorption frequencies used in the analysis.
E 932 Practice for Describing and Measuring Performance
For best results, the absorbance of the strongest bands should
of Dispersive Infrared Spectrometers
be in the range from 1 to 2, and several bands should have
E 1421 Practice for Describing and Measuring Performance
absorbances of 0.6 units or more. There are exceptions to this
of Fourier Transform Infrared (FT-IR) Spectrometers:
generalization based on the polarity of the molecules being
Level Zero and Level One
measured. For example, saturated hydrocarbons are nonpolar,
E 1642 Practice for General Techniques of Gas Chromatog-
2 and their identifying bands are not strong enough unless the
raphy Infrared (GC/IR) Analysis
−1
C-H stretch at 2920 cm is opaque and the deformation bands
3. Terminology are in the range from 1.5 to 2.0 absorbance units (A) at 1440
−1
to 1460 cm . Spectra with different amounts of sample in the
3.1 Definitions—For definitions of terms and symbols, refer
radiation path may be required to permit reliable analysis. If
to Terminology E 131.
spectra are to be identified by computerized curve matching,
4. Significance and Use
the absorbance of the strongest band should be less than 1;
otherwise, the effect of the instrument line shape function will
4.1 Infrared spectroscopy is the most widely used technique
cause errors in the relative intensities of bands in spectra
for identifying organic and inorganic materials. This practice
measured by dispersive spectrometers and by FT-IR spectrom-
eters with certain apodization functions (specially triangular).
This practice is under the jurisdiction of ASTM Committee E-13 on Molecular
5.2.1 Techniques for obtaining transmission spectra vary
Spectroscopy and is the direct responsibility of Subcommittee E13.03 on Infrared
Spectroscopy.
Current edition approved March 10, 1998. Published June 1998. Originally
e1 3
published as E 1252 – 88. Last previous edition E 1252 – 94 . The boldface numbers in parentheses refer to a list of references at the end of
Annual Book of ASTM Standards, Vol 03.06. the text.
Copyright © ASTM, 100 Barr Harbor Drive, West Conshohocken, PA 19428-2959, United States.
NOTICE: This standard has either been superceded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
E 1252
with the sample state. Most samples, except free-standing thin 6.4 Disposable IR Cards —These can be used to obtain
films, require IR transparent windows or matrices containing spectra of non-volatile liquids. A very small drop, usually less
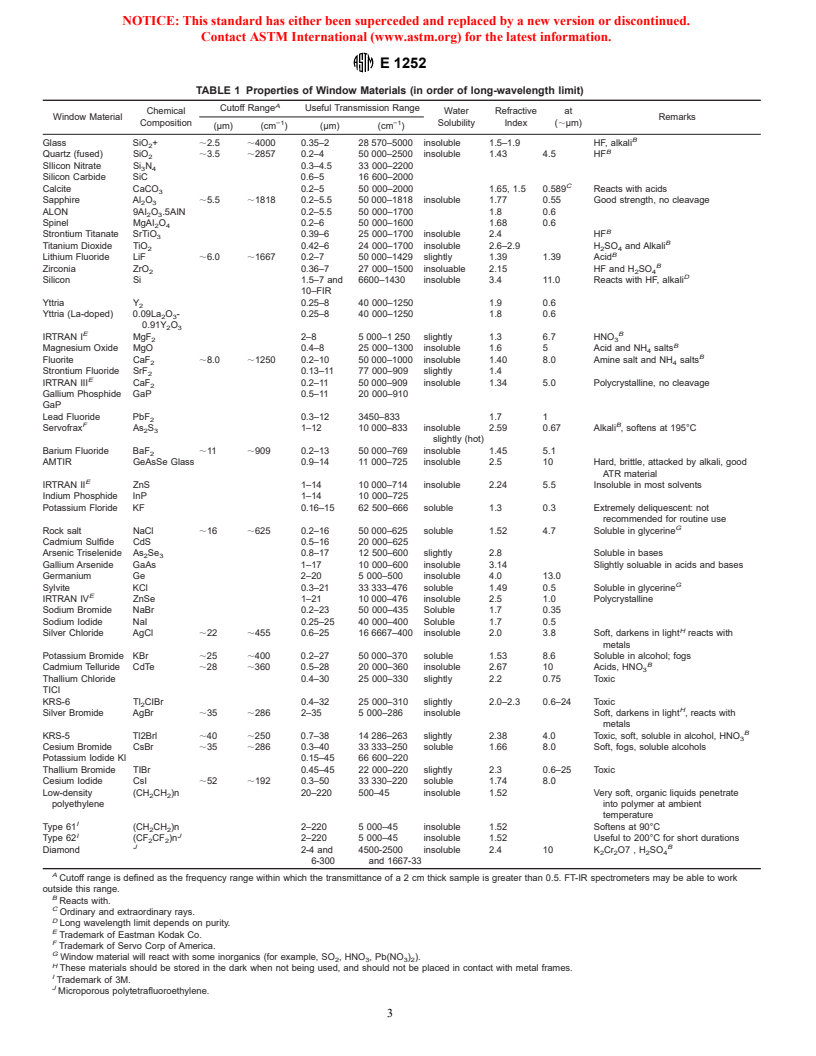
the sample. Table 1 gives the properties of IR window than 10 μL of the liquid, is applied near the edge of the sample
materials commonly employed. Selection of the window ma- application area. If the sample does not easily flow across the
terial depends on the region of the IR spectrum to be used for substrate surface, it may be spread using an appropriate tool.
analysis, on the absence of interference with the sample, and The sample needs to be applied in a thin layer, completely
adequate durability for the sample type. covering an area large enough that the entire radiation beam
5.3 Spectra obtained by reflection configurations commonly passes through the sample. Note that any volatile components
exhibit both reflection and absorption characteristics and are of a mixture will be lost in this process, which may make the
affected by the refractive indices of the media and the inter- use of a disposable card a poor choice for such systems.
faces. Spectral interpretation should be based on references run 6.5 Solution Techniques:
under the same experimental conditions. In particular, it should 6.5.1 Analysis of Materials Soluble in Infrared (IR) Trans-
be realized that the spectrum of the surface of a sample parent Solvent: The Split Solvent Technique—Many solid and
recorded by reflection will often differ from the spectrum of the liquid samples are soluble in solvents that are transparent in
bulk material as recorded by transmission spectroscopy. This is parts of the infrared spectral region. A list of solvents com-
because the chemistry of the surface often differs from that of monly used in obtaining solution spectra is given in Table 2.
the bulk, due to factors such as surface oxidation, migration of The selection of solvents depends on several factors. The
species from the bulk to the surface, and possible surface sample under examination must have adequate solubility, it
contaminants. Some surface measurements are extremely sen- must not react with the solvent, and the solvent must have
sitive to small amounts of materials present on a surface, appropriate transmission regions that enable a useful spectrum
whereas transmission spectroscopy is relatively insensitive to to be obtained. Combinations of solvents and window materi-
these minor components. als can often be selected that will allow a set of qualitative
5.3.1 Reflection spectra are obtained in four configurations: solution-phase spectra to be obtained over the entire IR region.
5.3.1.1 Specular reflectance (7.5), One example of this “split solvent” technique utilizes carbon
5.3.1.2 Diffuse reflectance (7.6), tetrachloride (CCl ) and carbon disulfide (CS ) as solvents.
4 2
5.3.1.3 Reflection-absorption (7.7),
NOTE 1—Warning: Both CCl and CS are toxic; keep in a well
4 2
5.3.1.4 Internal reflection (7.9). Refer to Practices E 573.
ventilated hood. Use of these solvents is prohibited in many laboratories.
This technique is also called Attenuated Total Reflection
In addition, CS is extremely flammable; keep away from ignition sources,
(ATR), and even a steam bath. Moreover, CS is reactive (sometimes violently) with
primary and secondary aliphatic amines and must not be used as a solvent
5.3.1.5 Grazing angle reflectance.
for these compounds. Similarly, CCl reacts with aluminum metal.
5.4 Photoacoustic IR spectra (11.2).
Depending on conditions such as temperature and particle size, the
5.5 Emission spectroscopy (11.4).
reaction has been lethally violent.
TEST METHODS AND TECHNIQUES 6.5.1.1 Absorption by CCl is negligible in the region
−1 −1
4000-1330 cm and by CS in the region 1330-400 cm in
6. Analysis of Liquids
cells of about 0.1 mm thickness. (Other solvents can be used.)
6.1 Fixed Cells—A wide range of liquid samples of low to Solutions are prepared, usually in the 5–10 % weight/volume
moderate viscosity may be introduced into a sealed fixed-path range, and are shaken to ensure uniformity. The solutions are
length cell. These are commercially available in a variety of transferred by clean pipettes or by syringes that have been
materials and path lengths. Typical path lengths are 0.01 to 0.2 cleaned with solvent and dried to avoid cross-contamination
with a previous sample. If the spectrum of a 10 % solution
mm. See 5.2 for considerations in selection of cell materials
and path lengths. contains many bands that are too deep and broad for accurate
frequency measurement, thinner cells or a more dilute solution
6.2 Capillary Films—Some liquids are too viscous to force
into or out of a sealed cell. Examination of viscous liquids is must be used.
accomplished by placing one or more drops in the center of a
NOTE 2—New syringes should be cleaned before use. Glass is the
flat window. Another flat window is then placed on top of the
preferred material. If plastic is used as containers, lids, syringes, pipettes,
liquid. Pressure is applied in order to form a bubble-free
and so forth, analytical blanks are necessary as a check against contami-
capillary film covering an area large enough that the entire nation.
radiation beam passes through the film. The film thickness is
6.5.1.2 A spectrum obtained by the split-solvent technique
regulated by the amount of pressure applied and the viscosity
in cells up to 0.5 to 1.0 mm-thickness, can be compensated for
of the liquid. A capillary film prepared in this manner has a
all solvent bands to yield the spectrum of only the sample
path length of about 0.01 mm. Volatile and highly fluid
itself. When a spectrometer that is capable of storing digital
materials may be lost from films prepared in this manner.
data is employed, the desired spectrum is obtained by a
Demountable spacers can be used when a longer path length is
computer-assisted subtraction of the stored data for the solvent
required to obtain a useful spectrum.
from the data for the solution. The user should refer to the
6.3 Internal Reflection Spectroscopy (IRS)—Viscous mate-
rials can be smeared on one or both sides of an internal
reflection element (IRE). See Practices E 573 for detailed
The 3M disposable IR Card is manufactured by 3M Co., Disposable Products
information on this technique. Division.
NOTICE: This standard has either been superceded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
E 1252
TABLE 1 Properties of Window Materials (in order of long-wavelength limit)
A
Cutoff Range Useful Transmission Range
Chemical Water Refractive at
Window Material Remarks
−1 −1
Composition Solubility Index (;μm)
(μm) (cm ) (μm) (cm )
B
Glass SiO + ;2.5 ;4000 0.35–2 28 570–5000 insoluble 1.5–1.9 HF, alkali
B
Quartz (fused) SiO ;3.5 ;2857 0.2–4 50 000–2500 insoluble 1.43 4.5 HF
SIlicon Nitrate Si N 0.3–4.5 33 000–2200
3 4
Silicon Carbide SiC 0.6–5 16 600–2000
C
Calcite CaCO 0.2–5 50 000–2000 1.65, 1.5 0.589 Reacts with acids
Sapphire Al O ;5.5 ;1818 0.2–5.5 50 000–1818 insoluble 1.77 0.55 Good strength, no cleavage
2 3
ALON 9AI O .5AIN 0.2–5.5 50 000–1700 1.8 0.6
2 3
Spinel MgAI O 0.2–6 50 000–1600 1.68 0.6
2 4
B
Strontium Titanate SrTiO 0.39–6 25 000–1700 insoluble 2.4 HF
B
Titanium Dioxide TiO 0.42–6 24 000–1700 insoluble 2.6–2.9 H SO and Alkali
2 2 4
B
Lithium Fluoride LiF ;6.0 ;1667 0.2–7 50 000–1429 slightly 1.39 1.39 Acid
B
Zirconia ZrO 0.36–7 27 000–1500 insoluable 2.15 HF and H SO
2 2 4
D
Silicon Si 1.5–7 and 6600–1430 insoluble 3.4 11.0 Reacts with HF, alkali
10–FIR
Yttria Y 0.25–8 40 000–1250 1.9 0.6
Yttria (La-doped) 0.09La O - 0.25–8 40 000–1250 1.8 0.6
2 3
0.91Y O
2 3
E B
IRTRAN I MgF 2–8 5 000–1 250 slightly 1.3 6.7 HNO
2 3
B
Magnesium Oxide MgO 0.4–8 25 000–1300 insoluble 1.6 5 Acid and NH salts
B
Fluorite CaF ;8.0 ;1250 0.2–10 50 000–1000 insoluble 1.40 8.0 Amine salt and NH salts
2 4
Strontium Fluoride SrF 0.13–11 77 000–909 slightly 1.4
E
IRTRAN III CaF 0.2–11 50 000–909 insoluble 1.34 5.0 Polycrystalline, no cleavage
Gallium Phosphide GaP 0.5–11 20 000–910
GaP
Lead Fluoride PbF 0.3–12 3450–833 1.7 1
F B
Servofrax As S 1–12 10 000–833 insoluble 2.59 0.67 Alkali , softens at 195°C
2 3
slightly (hot)
Barium Fluoride BaF ;11 ;909 0.2–13 50 000–769 insoluble 1.45 5.1
AMTIR GeAsSe Glass 0.9–14 11 000–725 insoluble 2.5 10 Hard, brittle, attacked by alkali, good
ATR material
E
IRTRAN II ZnS 1–14 10 000–714 insoluble 2.24 5.5 Insoluble in most solvents
Indium Phosphide InP 1–14 10 000–725
Potassium Floride KF 0.16–15 62 500–666 soluble 1.3 0.3 Extremely deliquescent: not
recommended for routine use
G
Rock salt NaCl ;16 ;625 0.2–16 50 000–625 soluble 1.52 4.7 Soluble in glycerine
Cadmium Sulfide CdS 0.5–16 20 000–625
Arsenic Triselenide As Se 0.8–17 12 500–600 slightly 2.8 Soluble in bases
2 3
Gallium Arsenide GaAs 1–17 10 000–600 insoluble 3.14 Slightly soluable in acids and bases
Germanium Ge 2–20 5 000–500 insoluble 4.0 13.0
G
Sylvite KCl 0.3–21 33 333–476 soluble 1.49 0.5 Soluble in glycerine
E
IRTRAN IV ZnSe 1–21 10 000–476 insoluble 2.5 1.0 Polycrystalline
Sodium Bromide NaBr 0.2–23 50 000–435 Soluble 1.7 0.35
Sodium Iodide NaI 0.25–25 40 000–400 Soluble 1.7 0.5
H
Silver Chloride AgCl ;22 ;455 0.6–25 16 6667–400 insoluble 2.0 3.8 Soft, darkens in light reacts with
metals
Potassium Bromide KBr ;25 ;400 0.2–27 50 000–370 soluble 1.53 8.6 Soluble in alcohol; fogs
B
Cadmium Telluride CdTe ;28 ;360 0.5–28 20 000–360 insoluble 2.67 10 Acids, HNO
Thallium Chloride 0.4–30 25 000–330 slightly 2.2 0
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.