ASTM D3352-03e1
(Test Method)Standard Test Method for Strontium Ion in Brackish Water, Seawater, and Brines
Standard Test Method for Strontium Ion in Brackish Water, Seawater, and Brines
SIGNIFICANCE AND USE
This test method5 can be used to determine strontium ions in brackish water, seawater, and brines.
SCOPE
1.1 This test method covers the determination of soluble strontium ion in brackish water, seawater, and brines by atomic absorption spectrophotometry.
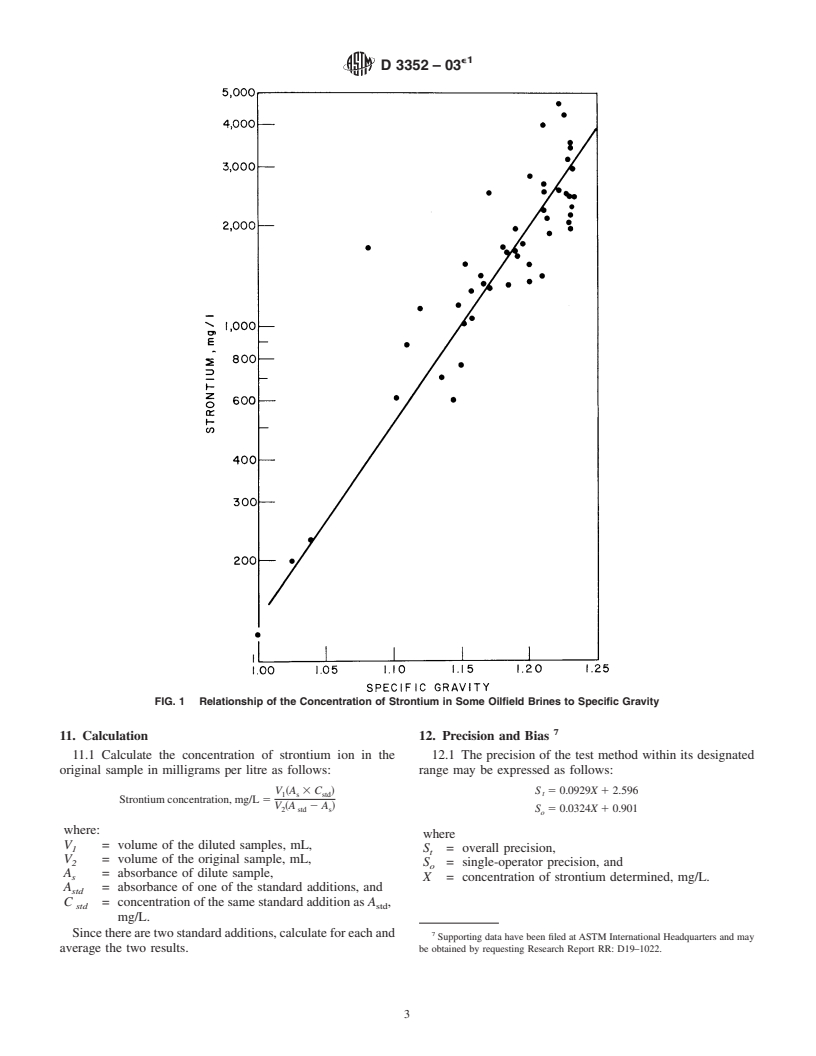
1.2 Samples containing from 5 to 2100 mg/L of strontium may be analyzed by this test method.
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
e1
Designation: D 3352 – 03
Standard Test Method for
1
Strontium Ion in Brackish Water, Seawater, and Brines
This standard is issued under the fixed designation D 3352; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1
e NOTE—Editorial corrections were made throughout in August 2003.
1. Scope* of the ground state element in the flame. A hollow cathode
lamp whose cathode is made of the element to be determined
1.1 This test method covers the determination of soluble
4
provides the radiation. The metal atoms to be measured are
strontium ion in brackish water, seawater, and brines by atomic
placed in the beam of radiation by aspirating the specimen into
absorption spectrophotometry.
an oxidant-fuel flame. A monochromator isolates the charac-
1.2 Samples containing from 5 to 2100 mg/L of strontium
teristic radiation from the hollow cathode lamp and a photo-
may be analyzed by this test method.
sensitive device measures the attenuated transmitted radiation.
1.3 This standard does not purport to address all of the
4.2 Sincethevariableandsometimeshighconcentrationsof
safety concerns, if any, associated with its use. It is the
matrix materials in the waters and brines affect absorption
responsibility of the user of this standard to establish appro-
differently,itisdifficulttopreparestandardssufficientlysimilar
priate safety and health practices and determine the applica-
to the waters and brines. To overcome this difficulty, the
bility of regulatory limitations prior to use.
method of additions is used in which three identical samples
2. Referenced Documents
are prepared and varying amounts of a standard added to two
of them. The three samples are then aspirated, the concentra-
2.1 ASTM Standards:
2
tion readings recorded, and the original sample concentration
D 1129 Terminology Relating to Water
2
calculated.
D 1193 Specification for Reagent Water
D 2777 Practice for Determination of Precision and Bias of
5. Significance and Use
2
Applicable Methods of Committee D19 on Water
5
5.1 This test method can be used to determine strontium
D 3370 Practices for Sampling Water from Closed Con-
2
ions in brackish water, seawater, and brines.
duits
2
D 5810 Guide for Spiking into Aqueous Samples
6. Interferences
D 5847 Practice for Writing Quality Control Specifications
3 6.1 The chemical suppression caused by silicon, aluminum,
for Standard Test Methods in Water Analysis
and phosphate is controlled by adding lanthanum. The lantha-
3. Terminology num also controls ionization interference.
3.1 Definitions—For definitions of terms used in this test
7. Apparatus
method, refer to Terminology D 1129.
7.1 Atomic Absorption Spectrophotometer—The instrument
4. Summary of Test Method shall consist of atomizer and burner, suitable pressure-
regulating devices capable of maintaining constant oxidant and
4.1 This test method is dependent on the fact that metallic
fuelpressureforthedurationofthetest,ahollowcathodelamp
elements, in the ground state, will absorb light of the same
for each metal to be tested, an optical system capable of
wavelength they emit when excited. When radiation from a
given excited element is passed through a flame containing
ground state atoms of that element, the intensity of the
4
transmitted radiation will decrease in proportion to the amount
For additional information on atomic absorption, see the following references:
Angino, E. E., and Billings, G. K., Atomic Absorption Spectrophotometry in
Geology, Elsevier Publishing Co., NewYork, N.Y., 1967. Dean, J.A., and Rains, T.
C., Editors, Flame Emission and Atomic Absorption Spectrometry Vol 1−Theory,
1
This test method is under the jurisdiction of ASTM Committee D19 on Water Marcel Dekker, New York, NY, 1969.
5
and is the direct responsibility of Subcommittee D19.05 on Inorganic Constituents Additional information is contained in the following references: Fletcher, G. F.,
in Water. and Collins, A. G., “Atomic Absorption Methods of Analysis of Oilfield Brines:
Current edition approved June 10, 2003. Published July 2003. Originally Barium,Calcium,Copper,Iron,Lead,Lithium,Magnesium,Manganese,Potassium,
approved in 1974. Last previous edition approved in 1999 as D 3352 – 94 (1999). Sodium, Strontium, and Zinc,” U.S. Bureau of Mines, Report of Investigations
2
Annual Book of ASTM Standards, Vol 11.01. 7861, 1974, 14 pp. Collins, A. G., Geochemistry of Oilfield Waters, Elsevier
3
Annual Book of ASTM Standards, Vol 11.02. Publishing Co., Amsterdam. The Netherlands, 1975.
*A Summary of Changes section appears at the end of this standard.
Copyright © ASTM International, 100
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.