ASTM D4658-09
(Test Method)Standard Test Method for Sulfide Ion in Water
Standard Test Method for Sulfide Ion in Water
SIGNIFICANCE AND USE
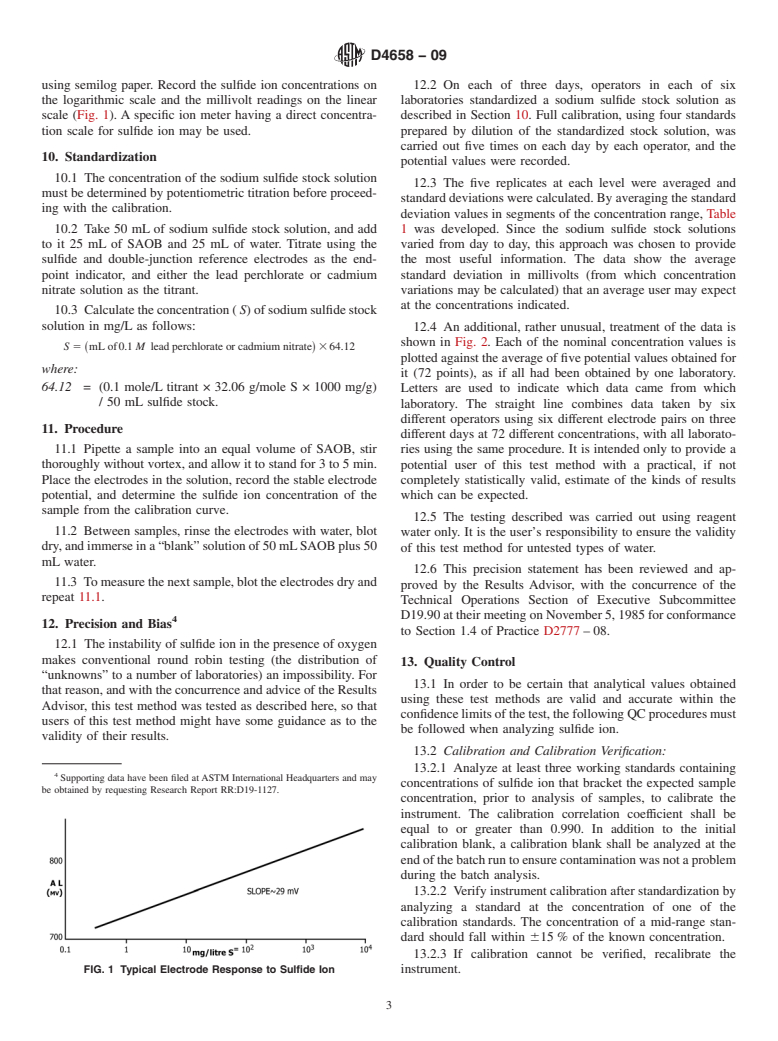
Sulfide ion is found in ground waters and wastewater, causing odor and corrosion problems. If acidified, these waters can release hydrogen sulfide, which is extremely toxic even at low levels. This test method provides a means for interference-free measurement of free sulfide ion.
Note 1—Sulfide forms complexes with hydrogen ions (HS1− and H2S). In addition, sulfide ion forms soluble complexes with elemental sulfur (S22−, S32−, S42−, etc.), tin, antimony, and arsenic ions.
SCOPE
1.1 This test method uses an ion-selective electrode to determine sulfide ion in water. The test method is applicable in the range from 0.04 to 4000 mg/L of sulfide.
1.2 Precision data presented in this test method were obtained using reagent water only. It is the user's responsibility to ensure the validity of this test method for untested types of water.
1.3 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use. Sulfide samples, when acidified, can release highly toxic hydrogen sulfide gas. For a specific precautionary statement, see Note 2.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation:D4658 −09
StandardTest Method for
1
Sulfide Ion in Water
This standard is issued under the fixed designation D4658; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope* 3. Terminology
1.1 This test method uses an ion-selective electrode to 3.1 Definitions: For definitions of terms used in this test
determinesulfideioninwater.Thetestmethodisapplicablein method, refer to Terminology D1129.
the range from 0.04 to 4000 mg/L of sulfide.
3.2 Definitions of Terms Specific to This Standard:
1.2 Precision data presented in this test method were ob- 3.2.1 For definitions of terms specific to this test method,
tainedusingreagentwateronly.Itistheuser’sresponsibilityto refer to Terminology D4127.
ensure the validity of this test method for untested types of
water. 4. Summary of Test Method
1.3 The values stated in SI units are to be regarded as 4.1 Sulfide ion is measured potentiometrically using a
standard. No other units of measurement are included in this sulfide ion-selective electrode in conjunction with a double-
standard. junction sleeve type reference electrode. Potentials are read
usingapHmeterhavinganexpandedmillivoltscalecapableof
1.4 This standard does not purport to address all of the
beingreadtothenearest0.1mV,oraspecificionmeterhaving
safety concerns, if any, associated with its use. It is the
a direct concentration scale for sulfide ion.
responsibility of the user of this standard to establish appro-
priate safety and health practices and determine the applica- 4.2 Samples are treated prior to analysis with sulfide anti-
bility of regulatory limitations prior to use. Sulfide samples,
oxidant buffer (SAOB). This buffer fixes the solution pH at a
when acidified, can release highly toxic hydrogen sulfide gas. highly alkaline level and contains ascorbic acid to retard air
For a specific precautionary statement, see Note 2.
oxidationofsulfideioninsolution.Thisensuresthatthesulfide
2− 1−
presentoccurschieflyasS ionratherthanascomplexedHS
2. Referenced Documents
or H S that are present at lower pH values.
2
2
2.1 ASTM Standards:
D1129Terminology Relating to Water 5. Significance and Use
D1193Specification for Reagent Water
5.1 Sulfide ion is found in ground waters and wastewater,
D2777Practice for Determination of Precision and Bias of
causing odor and corrosion problems. If acidified, these waters
Applicable Test Methods of Committee D19 on Water
can release hydrogen sulfide, which is extremely toxic even at
D3370Practices for Sampling Water from Closed Conduits
lowlevels.Thistestmethodprovidesameansforinterference-
D4127Terminology Used with Ion-Selective Electrodes
free measurement of free sulfide ion.
D5810Guide for Spiking into Aqueous Samples
1−
NOTE1—Sulfideformscomplexeswithhydrogenions(HS andH S).
2
D5847Practice for Writing Quality Control Specifications
In addition, sulfide ion forms soluble complexes with elemental sulfur
for Standard Test Methods for Water Analysis
2− 2− 2−
(S ,S ,S , etc.), tin, antimony, and arsenic ions.
2 3 4
1
This test method is under the jurisdiction ofASTM Committee D19 on Water 6. Apparatus
andarethedirectresponsibilityofSubcommitteeD19.05onInorganicConstituents
6.1 pH Meter, with expanded millivolt scale, or a specific
in Water.
Current edition approved May 15, 2009. Published May 2009. Originally ion meter having a direct concentration scale for sulfide ion.
approved in 1987. Last previous edition approved in 2008 as D4658–08. DOI:
6.2 Sulfide Ion-Selective Electrode .
10.1520/D4658-09.
2
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
6.3 Reference Electrode, double-junction sleeve type with
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
1.0 M potassium nitrate solution, pH adjusted to 13.5 with 1.0
Standards volume information, refer to the standard’s Document Summary page on
the ASTM website. M sodium hydroxide in the outer sleeve.
*A Summary of Changes section appears at the end of this standard
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
D4658−09
7. Reagents is as follows; 2 M NaOH; 0.2 M ascorbic acid, and 0.2 M
disodium EDTA (dihydrate).
7.1 Purity of Reagents—Reagent grade chemicals shall be
used in all tests. Unless otherwise indicated, it is intended that
NOTE 3—Freshly prepared SAOB, when stored in a tightly stoppered
bottle, has a shelf life of approximately two weeks, if opened frequently.
all reagents shall conform to the specifications of the Commit-
3
When oxidized, the solution turns d
...
This document is not anASTM standard and is intended only to provide the user of anASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
Designation:D4658–08 Designation: D 4658 – 09
Standard Test Method for
1
Sulfide Ion in Water
This standard is issued under the fixed designation D4658; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope Scope*
1.1 Thistestmethodusesanion-selectiveelectrodetodeterminesulfideioninwater.Thetestmethodisapplicableintherange
from 0.04 to 4000 mg/L of sulfide.
1.2 Precision data presented in this test method were obtained using reagent water only. It is the user’s responsibility to ensure
the validity of this test method for untested types of water.
1.3 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility
of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory
limitations prior to use.Sulfidesamples,whenacidified,canreleasehighlytoxichydrogensulfidegas.Foraspecificprecautionary
statement, see Note 2.
2. Referenced Documents
2
2.1 ASTM Standards:
D1129 Terminology Relating to Water
D1193 Specification for Reagent Water
D2777 Practice for Determination of Precision and Bias of Applicable Test Methods of Committee D19 on Water
D3370 Practices for Sampling Water from Closed Conduits
D4127 Terminology Used with Ion-Selective Electrodes
D5810 Guide for Spiking into Aqueous Samples
D5847 Practice for Writing Quality Control Specifications for Standard Test Methods for Water Analysis
3. Terminology
3.1 Definitions: For definitions of terms used in this test method, refer to Terminology D1129.
3.2 Definitions of Terms Specific to This Standard:
3.2.1 For definitions of terms specific to this test method, refer to Terminology D4127.
4. Summary of Test Method
4.1 Sulfide ion is measured potentiometrically using a sulfide ion-selective electrode in conjunction with a double-junction
sleeve type reference electrode. Potentials are read using a pH meter having an expanded millivolt scale capable of being read to
the nearest 0.1 mV, or a specific ion meter having a direct concentration scale for sulfide ion.
4.2 Samples are treated prior to analysis with sulfide antioxidant buffer (SAOB). This buffer fixes the solution pH at a highly
alkalinelevelandcontainsascorbicacidtoretardairoxidationofsulfideioninsolution.Thisensuresthatthesulfidepresentoccurs
2− 1−
chiefly as S ion rather than as complexed HS or H S that are present at lower pH values.
2
5. Significance and Use
5.1 Sulfide ion is found in ground waters and wastewater, causing odor and corrosion problems. If acidified, these waters can
release hydrogen sulfide, which is extremely toxic even at low levels. This test method provides a means for interference-free
measurement of free sulfide ion.
1− 2−
NOTE 1—Sulfide forms complexes with hydrogen ions (HS and H S). In addition, sulfide ion forms soluble complexes with elemental sulfur (S ,
2 2
2− 2−
S ,S , etc.), tin, antimony, and arsenic ions.
3 4
1
This test method is under the jurisdiction of ASTM Committee D19 on Water and are the direct responsibility of Subcommittee D19.05 on Inorganic Constituents in
Water.
Current edition approved Aug.May 15, 2008.2009. Published August 2008.May 2009. Originally approved in 1987. Last previous edition approved in 20032008 as
D4658–038.
2
ForreferencedASTMstandards,visittheASTMwebsite,www.astm.org,orcontactASTMCustomerServiceatservice@astm.org.For Annual Book of ASTM Standards
volume information, refer to the standard’s Document Summary page on the ASTM website.
*A Summary of Changes section appears at the end of this standard.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
1
---------------------- Page: 1 ----------------------
D4658–09
6. Apparatus
6.1 pH Meter, with expanded millivolt scale, or a specific ion meter having a direct concentration scale for sulfide ion.
6.2 Sulfide Ion-Selective Electrode .
6.3 Reference Electrode, double-junction sleeve type with 1.0 M potassium nitrate solution, pH adjusted to 13.5 with 1.0 M
sodium hydroxide in the outer sleeve.
7. Reagents
7.1 Purity of Reagents—Reagent grade chemicals shall be used in all tests. Unless otherwise indicated, it is intended
...









Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.