ASTM D4185-96(2001)e1
(Practice)Standard Practice for Measurement of Metals in Workplace Atmosphere by Flame Atomic Absorption Spectrophotometry
Standard Practice for Measurement of Metals in Workplace Atmosphere by Flame Atomic Absorption Spectrophotometry
SCOPE
1.1 This practice covers the collection, dissolution, and determination of trace metals in workplace atmospheres, by atomic absorption spectrophotometry.
1.2 The sensitivity, detection limit, and optimum working concentration for 23 metals are given in Table 1.
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use. (Specific safety precautionary statements are given in Section 9.)
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
e1
Designation:D4185–96 (Reapproved 2001)
Standard Practice for
Measurement of Metals in Workplace Atmosphere by Flame
Atomic Absorption Spectrophotometry
This standard is issued under the fixed designation D 4185; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
e NOTE—Keywords added to Section 14 editorially in October 2001.
1. Scope 3.2.3 working range for an analytical precision better than
3%—the range of sample concentrations that will absorb 10 to
1.1 This practice covers the collection, dissolution, and
70 % of the incident radiation (0.05 to 0.52 absorbance unit).
determination of trace metals in workplace atmospheres, by
atomic absorption spectrophotometry.
NOTE 1—Values for detection limit may vary from instrument to
1.2 The sensitivity, detection limit, and optimum working instrument.
concentration for 23 metals are given in Table 1.
4. Summary of Practice
1.3 This standard does not purport to address all of the
safety concerns, if any, associated with its use. It is the 4.1 The samples are collected on membrane filters and
treated with nitric acid to destroy the organic matrix and to
responsibility of the user of this standard to establish appro-
priate safety and health practices and determine the applica- dissolve the metals present. The analysis is subsequently made
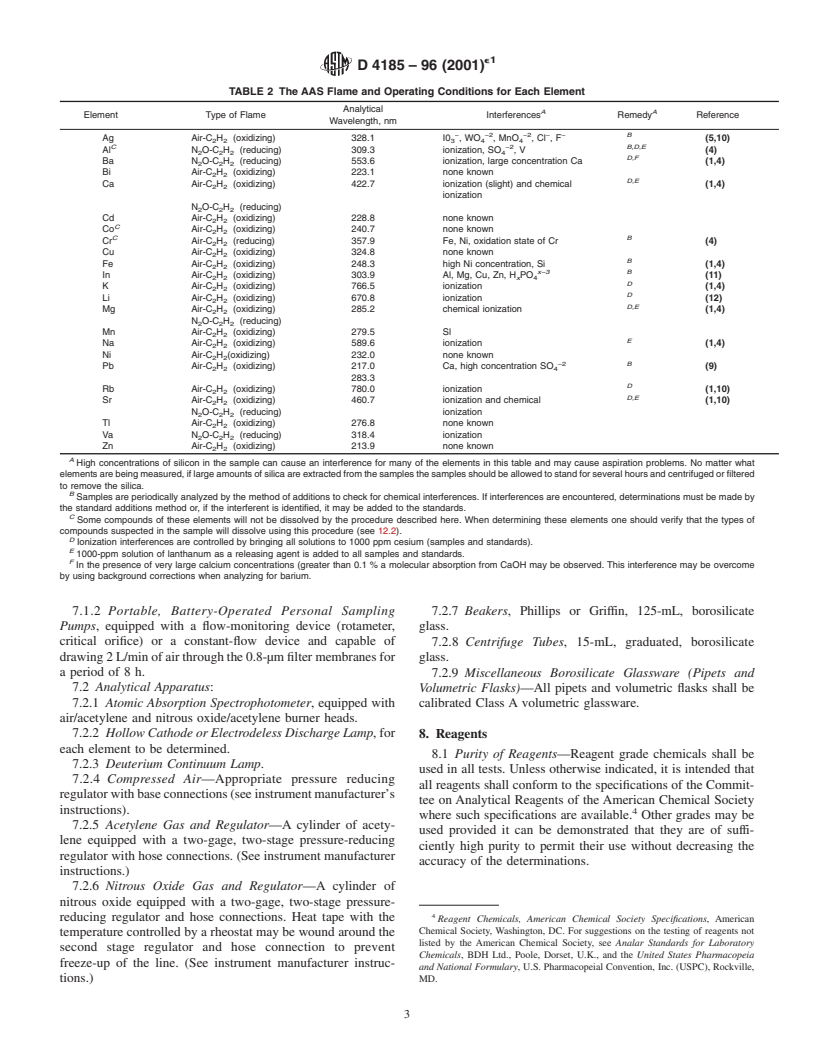
by flame atomic absorption spectrophotometry (AAS).
bility of regulatory limitations prior to use. (Specific safety
precautionary statements are given in Section 9.) 4.2 Samples and standards are aspirated into an appropriate
AAS flame. A hollow cathode or electrodeless discharge lamp
2. Referenced Documents
for the metal being determined provides a source of character-
2.1 ASTM Standards: istic radiation energy for that particular metal. The absorption
D 1193 Specification for Reagent Water of this characteristic energy by the atoms of interest in the
D 1356 Terminology Relating to Sampling and Analysis of flame is related to the concentration of the metal in the
Atmospheres aspirated sample. The flame and operating conditions for each
D 1357 Practice for Planning the Sampling of the Ambient element are listed in Table 2.
Atmosphere
5. Significance and Use
D 3195 Practice for Rotameter Calibration
5.1 Exposure to some metal-containing particulates has
3. Terminology
been demonstrated to cause dermatitis, skin ulcers, eye prob-
3.1 Definitions: lems, chemical pneumonitis, and other physical disorders (1).
3.1.1 For definitions of terms used in this practice, refer to 5.2 AAS is capable of quantitatively determining most
Terminology D 1356. metals in air samples at the levels required by federal, state,
3.2 Definitions of Terms Specific to This Standard: and local occupational health and air pollution regulations.
3.2.1 blank signal—that signal which results from all added
6. Interferences
reagents and a clean membrane filter ashed exactly as the
6.1 In AAS the occurrence of interferences is less common
samples.
3.2.2 detection limit—that concentration of a given element than in many other analytical techniques. Interferences can
occur, however, and when encountered are corrected for as
which produces a signal three times the standard deviation of
the blank signal. indicated in the following sections. The known interferences
and correction methods for each metal are indicated inTable 2.
The methods of standard additions and background monitoring
This practice is under the jurisdiction ofASTM Committee D22 onAir Quality
and correction (2-5) are used to identify the presence of an
and are the direct responsibility of Subcommittee D22.04 on Workplace Atmo-
spheres.
interference. Insofar as possible, the matrix of sample and
Current edition approved December 10, 1996. Published February 1997. Origi-
standard are matched to minimize the possible interference.
nally published as D 4185 – 90. Last previous edition D 4185 – 90.
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Standards volume information, refer to the standard’s Document Summary page on Boldface numbers in parentheses refer to the list of references appended to
the ASTM website. these methods.
Copyright ©ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA19428-2959, United States.
e1
D4185–96 (2001)
TABLE 1 Detection Limits and Optimum Working Concentration for 23 Metals
Detection Limit, µg/mL Optimum Linear Range
3 B
Element (approximately three times Upper Limit, TLV, mg/m (elements, compound classes, and oxides)
A
standard deviation) µg/mL
Ag 0.001 5 0.1 (metal) 0.01 (soluble compounds asAg)
Al 0.04 50 2.0 (soluble salts and alkyls not otherwise classified) 10 (metal dust and oxide)
5 (pyro powder and welding fume)
Ba 0.01 10 0.5 (soluble compounds)
Bi 0.03 10 No Limit expressed for this element
Ca 0.002 1 2 (oxide as CaO)
Cd 0.0008 1 0.01 (elemental and compounds—total dust)
0.002 (elemental compounds—respirable fraction)
Co 0.009 5 0.02 (elemental and inorganic) 0.1 (carbonyl and hydrocarbonyl)
Cr 0.003 5 0.5 (metal and Cr III compounds) 0.05 (water soluble Cr VI compounds)
0.01 (insoluble Cr VI compounds)
Cu 0.002 5 0.2 (fume) 1 (dust and mists as Cu)
Fe 0.005 5 5 (iron oxide fume) 5 (soluble salts as Fe)
In 0.03 50 0.1 (metal and compounds)
K 0.003 1 No Limit expressed for this element
Li 0.0008 1 No Limit expressed for this element
Mg 0.0002 0.5 10 (as MgO fume)
Mn 0.002 5 0.2 (elemental and inorganic compounds)
Na 0.0003 0.5 No Limit expressed for this element
Ni 0.006 5 0.05 (elemental, soluble and insoluble compounds)
Pb 0.02 10 0.15 (inorganic compounds, fume, dust)
Rb 0.003 5 No Limit expressed for this element
Sr 0.003 5 No Limit expressed for this element
Tl 0.02 50 0.1 (soluble compounds)
V 0.06 100 0.05 (pentoxide, respirable dust or fume, as V O )
2 5
Zn 0.002 1 10 (oxide dust as ZnO) 5 (oxide fume as ZnO)
A
Detection limit data and precision information supplied by Perkin-Elmer Corp., Norwalk, CT.
Note—Thesedetectionlimitsrepresentideallaboratoryconditions;variabilityduetosampling,digestion,reagents,andsamplehandlinghasnotbeentakenintoaccount.
B
Threshold Limit Values ofAirborne Contaminants and PhysicalAgents adopted byACGIH for 1994–1995. Values are elemental concentration except as noted.
6.2 Background or nonspecific absorption can occur from oflanthanumasareleasingelementminimizestheinterference
particles produced in the flame which can scatter light and from the formation of involatile compounds in the flame.
produce an apparent absorption signal. Light scattering may be Lanthanum forms involatile compounds preferentially with the
encountered when solutions of high salt content are being interferent so that the analyte stays free.
analyzed. They are most severe when measurements are made 6.6 Physical interferences may result if the physical prop-
at shorter wavelengths (for example, below about 250 nm). erties of the samples vary significantly. Changes in viscosity
Background absorption may also occur as the result of the and surface tension can affect the sample aspiration rate and
formation of various molecular species which can absorb light. thus cause erroneous results. Sample dilution or the method of
The background absorption can be accounted for by the use of standard additions, or both, are used to correct such interfer-
background correction techniques (2). ences. High concentrations of silica in the sample can cause
6.3 Spectral interferences are those interferences which aspiration problems. No matter what elements are being
result from an atom different from the one being measured that determined, if large amounts of silica are extracted from the
absorbs a portion of the radiation. Such interferences are samples they shall be allowed to stand for several hours and
extremely rare in AAS. In some cases multielement hollow centrifuged or filtered to remove the silica.
cathode lamps may cause a spectral interference by having 6.7 This procedure describes a generalized method for
closely adjacent emission lines from two different elements. In sample preparation which is applicable to the majority of
general, the use of multielement hollow cathode lamps is samples. There are some relatively rare chemical forms of a
discouraged. few of the elements listed in Table 1 that will not be dissolved
6.4 Ionization interference occurs when easily ionized at- bythisprocedure.Ifsuchchemicalformsaresuspected,results
oms are being measured. The degree to which such atoms are obtained using this procedure shall be compared with those
ionized is dependent upon the atomic concentration and the obtained using an appropriately altered dissolution procedure.
presenceofothereasilyionizedatoms.Thisinterferencecanbe Alternatively, the results may be compared with values ob-
controlled by the addition of a high concentration of another tained using a technique that does not require dissolving the
easily ionized element which will buffer the electron concen- sample (for example, X-ray fluorescence or activation analy-
tration in the flame. sis).
6.5 Chemical interferences occur in AAS when species
7. Apparatus
present in the sample cause variations in the degree to which
atoms are formed in the flame, or when different valence states 7.1 Sampling Apparatus:
of a single element have different absorption characteristics. 7.1.1 Cellulose Ester or Cellulose Nitrate Membrane Fil-
Such interferences may be controlled by adjusting the sample ters, with a pore size of 0.8 µm mounted in a 37-mm diameter
matrixorbythemethodofstandardadditions(3).Also,theuse two- or three-piece filter cassette.
e1
D4185–96 (2001)
TABLE 2 The AAS Flame and Operating Conditions for Each Element
Analytical
A A
Element Type of Flame Interferences Remedy Reference
Wavelength, nm
− −2 −2 − − B
Ag Air-C H (oxidizing) 328.1 I0 ,WO , MnO ,Cl ,F (5,10)
2 2 3 4 4
C −2 B,D,E
Al N O-C H (reducing) 309.3 ionization, SO ,V (4)
2 2 2 4
D,F
Ba N O-C H (reducing) 553.6 ionization, large concentration Ca (1,4)
2 2 2
Bi Air-C H (oxidizing) 223.1 none known
2 2
D,E
Ca Air-C H (oxidizing) 422.7 ionization (slight) and chemical (1,4)
2 2
ionization
N O-C H (reducing)
2 2 2
Cd Air-C H (oxidizing) 228.8 none known
2 2
C
Co Air-C H (oxidizing) 240.7 none known
2 2
C B
Cr Air-C H (reducing) 357.9 Fe, Ni, oxidation state of Cr (4)
2 2
Cu Air-C H (oxidizing) 324.8 none known
2 2
B
Fe Air-C H (oxidizing) 248.3 high Ni concentration, Si (1,4)
2 2
x−3 B
In Air-C H (oxidizing) 303.9 Al, Mg, Cu, Zn, H PO (11)
2 2 x 4
D
K Air-C H (oxidizing) 766.5 ionization (1,4)
2 2
D
Li Air-C H (oxidizing) 670.8 ionization (12)
2 2
D,E
Mg Air-C H (oxidizing) 285.2 chemical ionization (1,4)
2 2
N O-C H (reducing)
2 2 2
Mn Air-C H (oxidizing) 279.5 Sl
2 2
E
Na Air-C H (oxidizing) 589.6 ionization (1,4)
2 2
Ni Air-C H (oxidizing) 232.0 none known
2 2
−2 B
Pb Air-C H (oxidizing) 217.0 Ca, high concentration SO (9)
2 2 4
283.3
D
Rb Air-C H (oxidizing) 780.0 ionization (1,10)
2 2
D,E
Sr Air-C H (oxidizing) 460.7 ionization and chemical (1,10)
2 2
N O-C H (reducing) ionization
2 2 2
Tl Air-C H (oxidizing) 276.8 none known
2 2
Va N O-C H (reducing) 318.4 ionization
2 2 2
Zn Air-C H (oxidizing) 213.9 none known
2 2
A
High concentrations of silicon in the sample can cause an interference for many of the elements in this table and may cause aspiration problems. No matter what
elementsarebeingmeasured,iflargeamountsofsilicaareextractedfromthesamplesthesamplesshouldbeallowedtostandforseveralhoursandcentrifugedorfiltered
to remove the silica.
B
Samples are periodically analyzed by the method of additions to check for chemical interferences. If interferences are encountered, determinations must be made by
the standard additions method or, if the interferent is identified, it may be added to the standards.
C
Some compounds of these elements will not be dissolved by the procedure described here. When determining these elements one should verify that the types of
compounds suspected in the sample will dissolve using this procedure (see 12.2).
D
Ionization interferences are controlled by bringing all solutions to 1000 ppm cesium (samples and standards).
E
1000-ppm solution of lanthanum as a releasing agent is added to all samples and standards.
F
In the presence of very large calcium concentrations (greater than 0.1% a molecular absorption from CaOH may be observed. This interference may be overcome
by using background corrections when analyzing for barium.
7.1.2 Portable, Battery-Operated Personal Sampling 7.2.7 Beakers, Phillips or Griffin, 125-mL, borosilicate
Pumps, equipped with a flow-monitoring device (rotameter, glass.
critical orifice) or a constant-flow device and capable of 7.2.8 Centrifuge Tubes, 15-mL, graduated, borosilicate
drawing2L/minofairthroughthe0.8-µmfiltermembranesfor glass.
a period of 8 h. 7.2.9 Miscellaneous Borosilicate Glassware (Pipets and
7.2 Analytical Apparatus: Volumetric Flasks)—All pipets and volumetric flasks shall be
7.2.1 Atomic Absorption Spectrophotometer, equipped with
calibrated Class A volumetric glassware.
air/acetylene and nitrous oxide/acetylene burner heads.
7.2.2 HollowCathodeorElectrodelessDischargeLamp,for
8. Reagents
each element to be determined.
8.1 Purity of Reagents—Reagent grade chemicals shall be
7.2.3 Deuterium Continuum Lamp.
used in all tests. Unless otherwise indicated, it is intended that
7.2.4 Compressed Air—Appropriate pressure reducing
all reagents shall conform to the specifications of the Commit-
regulatorwithbaseconnections(seeinstrumentmanufacturer’s
tee on Analytical Reagents of the American Chemical Society
instructions). 4
where such specifications are available. Other grades may be
7.2.5 Acetylene Gas and Regulator—A cylinder of acety-
used provided it can be demonstrated that they are of suffi-
lene equipped with a two-gage, two-stage pressure-reducing
ciently high purity to permit their use without decreasing the
regulator with hose connections. (See instrument manufacturer
accuracy of the determinations.
instructions.)
7.2.6 Nitrous Oxide Gas and Regulator—A cylinder of
nitrous oxide equipped with a two-gage, two-stage pressure-
reducing regulator and hose connections. Heat tape with the
Reagent Chemicals, American Chemical Society Specifications, American
Chemical Society, Washington, DC. For suggestions on the testing of reagents not
temperature controlled by a rheostat may be wound around the
listed by the American Chemical Society, see Analar Standards f
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.