ASTM E1449-92(2000)
(Guide)Standard Guide for Supercritical Fluid Chromatography Terms and Relationships
Standard Guide for Supercritical Fluid Chromatography Terms and Relationships
SCOPE
1.1 This guide deals primarily with the terms and relationships used in supercritical fluid chromatography.
1.2 Since many of the basic terms and definitions also apply to gas chromatography and liquid chromatography, this guide is using, whenever possible, symbols identical to Practices E355 and E682.
General Information
Relations
Standards Content (Sample)
Designation: E 1449 – 92 (Reapproved 2000)
Standard Guide for
Supercritical Fluid Chromatography Terms and
Relationships
This standard is issued under the fixed designation E 1449; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope 3.4 In supercritical fluid chromatography, the temperature
may be constant, or changing during a chromatographic
1.1 This guide deals primarily with the terms and relation-
separation.
ships used in supercritical fluid chromatography.
3.4.1 Isothermal Supercritical Fluid Chromatography is
1.2 Since many of the basic terms and definitions also apply
the version of the technique in which the column temperature
to gas chromatography and liquid chromatography, this guide
is held constant during the passage of the sample components
is using, whenever possible, symbols identical to Practices
through the separation column.
E 355 and E 682.
3.4.2 Programmed Temperature Supercritical Fluid Chro-
2. Referenced Documents matography is the version of the technique in which the
column temperature is changed with time during the passage of
2.1 ASTM Standards:
the sample components through the separation column. Iso-
E 355 Practice for Gas Chromatography Terms and Rela-
thermal intervals may be included in the temperature program.
tionships
3.5 In supercritical fluid chromatography, the density may
E 682 Practice for Liquid Chromatography Terms and Re-
be constant or changing during the chromatographic separa-
lationships
tion.
3. Names of Techniques
3.5.1 Isoconfertic is a term used when the density of the
mobile phase is kept constant for a specified time or for the
3.1 Supercritical Fluid Chromatography, abbreviated as
entire chromatographic separation.
SFC, comprises all chromatographic methods in which both
3.5.2 Programmed Density Supercritical Fluid Chromatog-
the mobile phase is supercritical under the conditions of
raphy is the version of the technique in which the column
analysis and where the solvating properties of the fluid have a
density is changed with time during the passage of the sample
measurable affect on the separation. Early work in the field was
components through the separation column. Isoconfertic inter-
performed under a broader heading–dense gas chromatogra-
vals may be included in the density program.
phy. Related work in the field uses subcritical or near-critical
3.5.3 Flow Programming is a technique where the mobile
conditions to affect separation.
phase linear velocity is changed during the chromatographic
3.2 Separation is achieved by differences in the distribution
procedure. However, with fixed orifice restrictors, flow pro-
of the components of a sample between the mobile and
gramming is more complex requiring an increase in pressure to
stationary phases, causing them to move through the column at
effect an increase in linear velocity.
different rates (differential migration).
3.6 In supercritical fluid chromatography, the composition
3.3 In supercritical fluid chromatography, the pressure may
of the mobile phase may be constant or changing during a
be constant or changing during a chromatographic separation.
chromatographic separation.
3.3.1 Isobaric is a term used when the mobile phase is kept
3.6.1 The term Isocratic is used when the composition of
at constant pressure. This may be for a specified time interval
the mobile phase is kept constant during a chromatographic
or for the entire chromatographic separation.
separation.
3.3.2 Programmed Pressure Supercritical Fluid Chroma-
3.6.2 The term Gradient Elution is used to specify the
tography is the version of the technique in which the column
technique when a deliberate change in the mobile phase
pressure is changed with time during the passage of the sample
composition is made during the chromatographic procedure.
components through the separation column. Isobaric intervals
Isocratic intervals may be included in the gradient program.
may be included in the pressure program.
4. Apparatus
4.1 Pumps—The function of the pumps is to deliver the
This guide is under the jurisdiction of ASTM Committee E13 on Molecular
Spectroscopy and is the direct responsibility of Subcommittee E13.19 on Chroma-
mobile phase at a controlled flow rate to the chromatographic
tography.
column.
Current edition approved Jan. 15, 1992. Published March 1992.
2 4.1.1 Syringe Pumps have a piston that advances at a
Annual Book of ASTM Standards, Vol 14.01.
Copyright © ASTM, 100 Barr Harbor Drive, West Conshohocken, PA 19428-2959, United States.
E 1449
controlled rate within a smooth cylinder to displace the mobile tors (for example, UV).
phase. 4.4.1 A Linear Restrictor is a length of small i.d. tubing of
uniform bore. Linear restrictors are made of polyimidecoated
4.1.2 Reciprocating Pumps have a single or dual chamber
fused silica tubing, or stainless steel or other tubing of the
from which mobile phase is displaced by reciprocating pis-
appropriate diameter. The amount of restriction provided is
ton(s) or diaphragm(s).
dependent upon both the length and i.d. of the tubing.
4.2 Sample Inlet Systems represent the means for introduc-
4.4.2 A Tapered Restrictor is a length of small i.d. tubing
ing samples into the columns.
where one end has been reduced by drawing in a flame in the
4.2.1 Direct Injection is a sample introduction technique
case of fused silica tubing, or crimped in the case of metal
whereby the entire volume of sample is swept onto the head of
tubing.
the analytical column. Its use is most prevalent in packed
4.4.3 An Integral Restrictor (1) consists of a length of
column SFC.
fused silica tubing with one end closed by heating with a
4.2.2 Split-Flow Injection introduces only a portion of the
microtorch. This closed end is then ground until a hole with the
sample volume onto the analytical column so as to prevent
desired initial linear velocity is obtained.
overloading of the column in open tubular SFC. This is
4.4.4 A Converging-Diverging Restrictor (2) has the wall of
achieved by the use of a splitter tee or similar contrivance, such
the tubing collapsed slightly near one end forming a constric-
that the incoming slug of sample is divided between the
tion. This constriction is similar to a venturi in profile and the
analytical column and a flow restrictor vented to waste. The
point of smallest diameter is located about 1 to 2 mm from the
amount of sample deposited on the column is a function of the
end of the tubing.
ratio of the flow to the column versus the flow through this
4.4.5 An Orifice is a type of restrictor which uses a metal
restrictor. This ratio can thus be adjusted for different samples
disk or diaphragm with an appropriately sized opening. This
and column capacities.
type normally requires an adapter or holder specifically de-
4.2.3 Timed-Split (Moving-Split) Injection achieves the
signed to couple the device to a detector.
same end result as split-flow injection. The volume of sample
4.4.6 A Porous Frit Restrictor consists of a length of fused
introduced onto the column is governed by the rapid back-and-
silica tubing containing a porous plug at one end.
forth motion of an internal-loop sample rotor in a valve. The
4.4.7 A Back Pressure Regulator consists of a diaphragm
time interval between the two motions determines the volume
valve which can be adjusted to control the pressure maintained
of sample injected, with shorter times delivering smaller
on its inlet (instrument) side. The outlet discharge pressure is
volumes.
nominally one atmosphere.
4.2.4 On-Line Supercritical Extraction is a means of di-
4.5 Detectors are devices that respond to the presence of
rectly introducing a sample or portion of a sample into a
eluted solutes in the mobile phase emerging from the column.
supercritical fluid chromatograph. The sample is placed in an
Ideally, the response should be proportional to the mass or
extraction cell and extracted with the supercritical fluid. The
concentration of solute in the mobile phase. Detectors may be
extraction effluent containing the solutes of interest are ulti-
divided either according to the type of measurement or the
mately transferred to the column by the action of switching or
principle of detection.
sampling valves. This can be accomplished with or without
4.5.1 Differential Concentration Detectors measure the
solute focusing (that is, using a suitable trap such as a
proportion of eluted sample component(s) in the mobile phase
cryogenic trapping).
passing through the detector. The peak area is inversely
4.3 Columns consist of tubes that contain the stationary
proportional to the mobile phase flow rate.
phase and through which the supercritical fluid mobile phase
4.5.2 Differential Mass Detectors measure the instanta-
flows.
neous mass of a component within the detector per unit time
4.3.1 Packed Column Supercritical Fluid Chromatography-
(g/s). The area under the curve is independent of the mobile
uses an active solid or a liquid that is chemically bonded to a
phase flow rate.
solid and packed into a column, generally stainless steel or
fused silica; as the stationary phase. 5. Reagents
4.3.2 Wall-Coated Open-Tubular Supercritical Fluid Chro-
5.1 Supercritical Fluid is a fluid state of a substance inter-
matography uses a liquid that is chemically bonded to the wall
mediate between a gas and a liquid. A supercritical fluid may
of an open-tubular column as stationary phase. Fused silica
be defined from the accompanying phase diagram (Fig. 1). The
tubing columns, internal diameter (i.d.) > 100 μm, may shatter
supercritical fluid region is defined by temperatures and
at pressures employed in SFC. A high degree of crosslinking is
pressures, both above the critical values. A subcritical fluid (or
desirable to reduce stationary phase solubility in the mobile
liquid) is a compound that would usually be a gas at ambient
phase.
temperature but is held as a liquid by the application of
4.4 Restrictors are devices employed to maintain the pres-
pressure below its supercritical point.
sure in the chromatographic system. The pressure of the
5.1.1 The Critical Temperature is the temperature above
supercritical fluid is usually reduced to ambient after passage
through the restrictor. The mobile phase flow rate is determined
The boldface numbers in parentheses refer to the list of references at the end
by the restrictor dimensions or operation. The restrictor is
of this guide.
placed before some types of detectors (for example, flame 4
Cortez, H., Pfeiffer, C., Richter, B., and Stevens, T. U. S., Patent No. 4 793 920,
ionization, mass spectrometer) and after other types of detec- 1988.
E 1449
5.5 The Column Packing consists of all the material used to
fill packed columns, including the solid support and the bonded
phase or the interactive solid.
5.6 Solutes are the sample components that are introduced
into the chromatographic system and are transported by the
mobile phase and elute through the column. Some solutes may
be unretained.
6. Readout
6.1 A Chromatogram is a plot of detector response against
time or effluent volume. Idealized chromatograms obtained
with a differential detector for an unretained substance and one
other component are shown in Fig. 2.
6.2 The definitions in 6.2.1-6.2.6 apply to chromatograms
obtained directly by means of differential detectors or indi-
rectly by differentiating the response of integral detectors.
6.2.1 A Baseline is that portion of a chromatogram where no
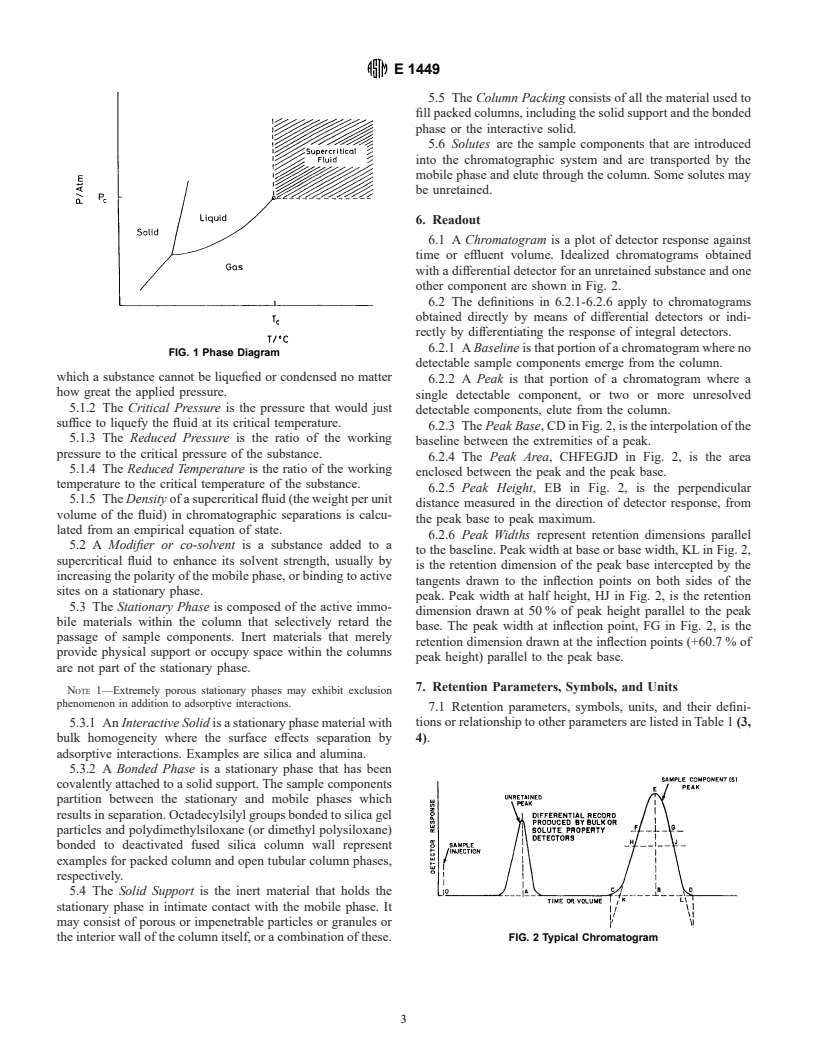
FIG. 1 Phase Diagram
detectable sample components emerge from the column.
which a substance cannot be liquefied or condensed no matter
6.2.2 A Peak is that portion of a chromatogram where a
how great the applied pressure.
single detectable component, or two or more unresolved
5.1.2 The Critical Pressure is the pressure that would just
detectable components, elute from the column.
suffice to liquefy the fluid at its critical temperature.
6.2.3 The Peak Base, CD in Fig. 2, is the interpolation of the
5.1.3 The Reduced Pressure is the ratio of the working
baseline between the extremities of a peak.
pressure to the critical pressure of the substance.
6.2.4 The Peak Area, CHFEGJD in Fig. 2, is the area
5.1.4 The Reduced Temperature is the ratio of the working
enclosed between the peak and the peak base.
temperature to the critical temperature of the substance.
6.2.5 Peak Height, EB in Fig. 2, is the perpendicular
5.1.5 The Density of a supercritical fluid (the weight per unit
distance measured in the direction of detector response, from
volume of the fluid) in chromatographic separations is calcu-
the peak base to peak maximum.
lated from an empirical equation of state.
6.2.6 Peak Widths represent retention dimensions parallel
5.2 A Modifier or co-solvent is a substance added to a
to the baseline. Peak width at base or base width, KL in Fig. 2,
supercritical fluid to enhance its solvent strength, usually by
is the retention dimension of the peak base intercepted by the
increasing the polarity of the mobile phase, or binding to active
tangents drawn to the inflection points on both sides of the
sites on a stationary phase.
peak. Peak width at half height, HJ in Fig. 2, is the retention
5.3 The Stationary Phase is composed of the active immo-
dimension drawn at 50 % of peak height parallel to the peak
bile materials within the column that selectively retard the
base. The peak width at inflection point, FG in Fig. 2, is the
passage of sample components. Inert materials that merely
retention dimension drawn at the inflection points (+60.7 % of
provide physical support or occupy space within the columns
peak height) parallel to the peak base.
are not part of the stationary phase.
7. Retention Parameters, Symbols, and Units
NOTE 1—Extremely porous stationary phases may exhibit exclusion
phenomenon in addition to adsorptive interactions.
7.1 Retention parameters, symbols, units, and their defini-
tions or relationship to other parameters are listed in Table 1 (3,
5.3.1 An Interactive Solid is a stationary phase material with
bulk homogeneity where the surface effects separation by 4).
adsorptive interactions. Examples are silica and alumina.
5.3.2 A Bonded Phase is a stationary phase that has been
covalently attached to a solid support. The sample components
partition between the stationary and mobile phases which
results in separation. Octadecylsilyl groups bonded to silica gel
particles and polydimethylsiloxane (or dimethyl polysiloxane)
bonded to deactivated fused silica column wall represent
examples for packed column and open tubular column phases,
respectively.
5.4 The Solid Support is the inert material that holds the
stationary phase in intimate contact with the mobile phase
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.