ASTM E265-07(2013)
(Test Method)Standard Test Method for Measuring Reaction Rates and Fast-Neutron Fluences by Radioactivation of Sulfur-32
Standard Test Method for Measuring Reaction Rates and Fast-Neutron Fluences by Radioactivation of Sulfur-32
SIGNIFICANCE AND USE
5.1 Refer to Guides E720 and E844 for the selection, irradiation, and quality control of neutron dosimeters.
5.2 Refer to Practice E261 for a general discussion of the determination of fast-neutron fluence and fluence rate with threshold detectors.
5.3 The activation reaction produces 32P, which decays by the emission of a single beta particle in 100 % of the decays, and which emits no gamma rays. The half life of 32P is 14.262 (14)3 days (1) 4 and the maximum beta energy is 1710 keV (2).
5.4 Elemental sulfur is readily available in pure form and any trace contaminants present do not produce significant amounts of radioactivity. Natural sulfur, however, is composed of 32S (95.02 % (9)), 34S (4.21 % (8)) (1), and trace amounts of other sulfur isotopes. The presence of these other isotopes leads to several competing reactions that can interfere with the counting of the 1710-keV beta particle. This interference can usually be eliminated by the use of appropriate techniques, as discussed in Section 8.
SCOPE
1.1 This test method describes procedures for measuring reaction rates and fast-neutron fluences by the activation reaction 32S(n,p)32P.
1.2 This activation reaction is useful for measuring neutrons with energies above approximately 3 MeV.
1.3 With suitable techniques, fission-neutron fluences from about 5 × 108 to 1016 n/cm 2 can be measured.
1.4 Detailed procedures for other fast-neutron detectors are described in Practice E261.
1.5 This standard does not purport to address all of the safety problems, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: E265 − 07(Reapproved 2013)
Standard Test Method for
Measuring Reaction Rates and Fast-Neutron Fluences by
Radioactivation of Sulfur-32
This standard is issued under the fixed designation E265; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
This standard has been approved for use by agencies of the U.S. Department of Defense.
1. Scope E844Guide for Sensor Set Design and Irradiation for
Reactor Surveillance, E 706 (IIC)
1.1 This test method describes procedures for measuring
E944Guide for Application of Neutron Spectrum Adjust-
reaction rates and fast-neutron fluences by the activation
32 32 ment Methods in Reactor Surveillance, E 706 (IIA)
reaction S(n,p) P.
E1018Guide for Application of ASTM Evaluated Cross
1.2 Thisactivationreactionisusefulformeasuringneutrons
Section Data File, Matrix E706 (IIB)
with energies above approximately 3 MeV.
3. Terminology
1.3 With suitable techniques, fission-neutron fluences from
8 16 2
about 5×10 to 10 n/cm can be measured.
3.1 Definitions:
3.1.1 Refer to Terminology E170.
1.4 Detailed procedures for other fast-neutron detectors are
described in Practice E261.
4. Summary of Test Method
1.5 This standard does not purport to address all of the
safety problems, if any, associated with its use. It is the 4.1 Elemental sulfur or a sulfur-bearing compound is irra-
diatedinaneutronfield,producingradioactive Pbymeansof
responsibility of the user of this standard to establish appro-
32 32
priate safety and health practices and determine the applica- the S(n,p) P activation reaction.
bility of regulatory limitations prior to use.
4.2 The beta particles emitted by the radioactive decay of
ParecountedbytechniquesdescribedinMethodsE181and
2. Referenced Documents
thereactionrate,asdefinedinPracticeE261,iscalculatedfrom
2.1 ASTM Standards:
the decay rate and irradiation conditions.
E170Terminology Relating to Radiation Measurements and
4.3 The neutron fluence above 3 MeV can then be calcu-
Dosimetry
lated from the spectral-averaged neutron activation cross
E181Test Methods for Detector Calibration andAnalysis of
section, σ¯, as defined in Practice E261.
Radionuclides
E261Practice for Determining Neutron Fluence, Fluence
5. Significance and Use
Rate, and Spectra by Radioactivation Techniques
5.1 Refer to Guides E720 and E844 for the selection,
E720Guide for Selection and Use of Neutron Sensors for
irradiation, and quality control of neutron dosimeters.
Determining Neutron Spectra Employed in Radiation-
Hardness Testing of Electronics
5.2 Refer to Practice E261 for a general discussion of the
E721Guide for Determining Neutron Energy Spectra from
determination of fast-neutron fluence and fluence rate with
Neutron Sensors for Radiation-Hardness Testing of Elec-
threshold detectors.
tronics
5.3 The activation reaction produces P, which decays by
the emission of a single beta particle in 100% of the decays,
and which emits no gamma rays. The half life of P is 14.262
ThistestmethodisunderthejurisdictionofASTMCommitteeE10onNuclear
3 4
Technology and Applicationsand is the direct responsibility of Subcommittee (14) days (1) andthemaximumbetaenergyis1710keV (2).
E10.07 on Radiation Dosimetry for Radiation Effects on Materials and Devices.
Current edition approved Jan. 1, 2013. Published January 2013. Originally
ε1
approved in 1970. Last previous edition approved in 2007 as E265–07 . DOI:
10.1520/E0265-07R13. The non-bolface number in parentheses after the nuclear data indicates the
For referenced ASTM standards, visit the ASTM website, www.astm.org, or uncertainty in the last significant digit of the preceding number. For example, 8.1 s
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM (5) means 8.1 6 0.5 seconds.
Standards volume information, refer to the standard’s Document Summary page on Theboldfacenumbersinparenthesesrefertothelistofreferencesattheendof
the ASTM website. this test method.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
E265 − 07 (2013)
5.4 Elemental sulfur is readily available in pure form and since high temperatures are usually associated with a high-
any trace contaminants present do not produce significant neutron fluence rate. The sulfur content by weight of
amounts of radioactivity. Natural sulfur, however, is composed (NH ) SO is 24%, of Li SO is 29.2%, and of MgSO is
4 2 4 2 4 4
32 34
of S(95.02%(9)), S(4.21%(8)) (1),andtraceamountsof 26.6%.
othersulfurisotopes.Thepresenceoftheseotherisotopesleads 32
7.3 The isotopic abundance of S in natural sulfur is 95.02
to several competing reactions that can interfere with the
6 0.09 atom% (1).
counting of the 1710-keV beta particle. This interference can
usually be eliminated by the use of appropriate techniques, as
8. Sample Preparation and Irradiation
discussed in Section 8.
8.1 Place sulfur in pellet or powdered form in a uniform
fast-neutron flux for a predetermined period of time. Record
6. Apparatus
32 the beginning and end of the irradiation period.
6.1 Since only beta particles of P are counted, propor-
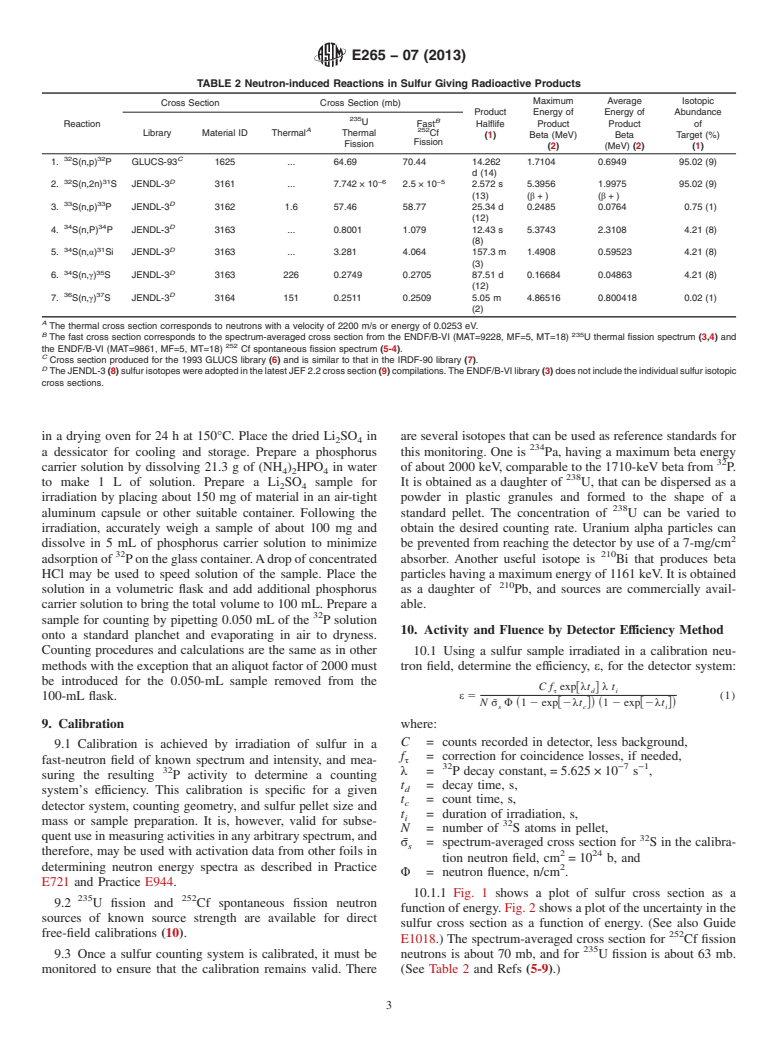
tional counters or scintillation detectors can be used. Because 8.2 Table 2 lists competing reaction products that must be
of the high resolving time associated with Geiger-Mueller eliminated from the counting. Those resulting from thermal-
33 35 37
counters,theiruseisnotrecommended.Theycanbeusedonly neutroncapture,thatis, P, S,and S,canbereducedbythe
with relatively low counting rates, and then only if reliable irradiation of the sulfur inside 1 mm-thick cadmium shields.
corrections for coincidence losses are applied. This should be done whenever possible in thermal-neutron
environments. Those reaction products having relatively short
6.2 RefertoMethodsE181forpreparationofapparatusand
31 34 31 37
half-lives, that is, S, P, Si, and S, can be eliminated by
counting procedures.
a waiting period before the counting is started.Adelay of 24 h
is sufficient for the longest lived of these, although shorter
7. Materials and Manufacture
delays are possible depending on the degree of thermalization
7.1 Commercially available sublimed flowers of sulfur are
of the neutron field. Finally, those with relatively low beta
inexpensive and sufficiently pure for normal usage. Sulfur can
33 35
particle energies, that is, Pand S, can be eliminated by the
be used directly as a powder or pressed into pellets. Sulfur
inclusion of a 70-mg/cm aluminum absorber in front of the
pelletsarenormallymadeatleast3mmthickinordertoobtain
detector.Forparticularlylongdecaytimes,anabsorbermustbe
maximum counting sensitivity independent of small variations
used because the S becomes dominant. Note that the use of
in pellet mass. A 0.8 g/cm pellet can be considered infinitely
an internal (windowless) detector maximizes the interference
thickforthemostenergeticbetaparticlefrom P(seeTable1).
in counting from S.
Due to the relatively long half-life of P, it may not be
8.3 Irradiated sulfur can be counted directly, or may be
practicaltouseapelletmorethanonce.Aperiodofatleastone
burned to increase the efficiency of the counting system.
year is recommended between uses. However, see 8.2 regard-
Dilution may be used to reduce counting system efficiency for
ing long-lived interfering reaction products.
measurements of high neutron fluences.
7.2 Where temperatures approaching the melting point of
8.4 Burning the sulfur leaves a residue of P that can be
sulfur are encountered (113°C), sulfur-bearing compounds
counted without absorption of the beta particles in the sulfur
such as ammonium sulfate (NH ) SO , lithium sulfate Li SO ,
4 2 4 2 4
pellet. Place the sulfur in an aluminum planchet on a hot plate
or magnesium sulfate MgSO can be used. These are suitable
until the sulfur melts and turns to a dark amber color. At this
fortemperaturesupto250,850,and1000°C,respectively.The
point the liquid gives off sulfur fumes. Ignite the fumes by
reduced sensitivity of these compounds offers no disadvantage
bringing a flame close to the dish, and allow the sulfur to burn
out completely. In order to reduce the sputtering that can lead
TABLE 1 Sulfur Counting Rate Versus Mass for a Pellet of
to variations in the amount of P remaining on the planchet,
25.4-mm Diameter
thehotplatemustbeonlyashotasnecessarytomeltthesulfur.
Sample Mass, g Relative Counting Rate
In addition, air flow to the burning sulfur must be controlled,
0.4 0.46
suchasbytheplacementofachimneyaroundthesulfur.Count
0.6 0.58
the residue remaining on the dish for beta activity.
0.8 0.66
1.0 0.73
NOTE 1—The fumes given off by the burning sulfur are toxic. Burning
1.2 0.78
should be done under a ventilating hood.
1.4 0.82
1.6 0.86
8.5 An alternative to burning is sublimation of the sulfur
1.8 0.89
under a heat lamp. Removal of the sulfur is very gradual, and
2.0 0.91
2.2 0.93
there is no loss of P from sputtering.
2.4 0.94
2.6 0.95 8.6 Counting of dilute samples is useful for measuring high
2.8 0.96
neutron fluences, although it is applicable to virtually all
3.0 0.97
irradiation conditions. Use lithium sulfate, reagent grade or
3.2 0.98
3.4 0.99 better, as the target material because of its high melting point
3.6 0.99
(860°C), good solubility in water, and minimum production of
3.8 1.0
undesirable activation products. Prepare a dry powder by
4.0 1.0
spreading about 10 g of Li SO in a weighing bottle and place
2 4
E265 − 07 (2013)
TABLE 2 Neutron-induced Reactions in Sulfur Giving Radioactive Products
Maximum Average Isotopic
Cross Section Cross Section (mb)
Product Energy of Energy of Abundance
B
U
Reaction Fast Halflife Product Product of
A 252
Library Material ID Thermal Thermal Cf
(1) Beta (MeV) Beta Target (%)
Fission
Fission
(2) (MeV) (2) (1)
32 32 C
1. S(n,p) P GLUCS-93 1625 . 64.69 70.44 14.262 1.7104 0.6949 95.02 (9)
d (14)
32 31 D −6 −5
2. S(n,2n) S JENDL-3 3161 . 7.742 × 10 2.5×10 2.572 s 5.3956 1.9975 95.02 (9)
(13) (β+) (β+)
33 33 D
3. S(n,p) P JENDL-3 3162 1.6 57.46 58.77 25.34 d 0.2485 0.0764 0.75 (1)
(12)
34 34 D
4. S(n,P) P JENDL-3 3163 . 0.8001 1.079 12.43 s 5.3743 2.3108 4.21 (8)
(8)
34 31 D
5. S(n,α) Si JENDL-3 3163 . 3.281 4.064 157.3 m 1.4908 0.59523 4.21 (8)
(3)
34 35 D
6. S(n,γ) S JENDL-3 3163 226 0.2749 0.2705 87.51 d 0.16684 0.04863 4.21 (8)
(12)
36 37 D
7. S(n,γ) S JENDL-3 3164 151 0.2511 0.2509 5.05 m 4.86516 0.800418 0.02 (1)
(2)
A
The thermal cross section corresponds to neutrons with a velocity of 2200 m/s or energy of 0.0253 eV.
B 235
The fast cross section corresponds to the spectrum-averaged cross section from the ENDF/B-VI (MAT=9228, MF=5, MT=18) U thermal fission spectrum (3,4) and
the ENDF/B-VI (MAT=9861, MF=5, MT=18) Cf spontaneous fission spectrum (5-4).
C
Cross section produced for the 1993 GLUCS library (6) and is similar to that in the IRDF-90 library (7).
D
TheJENDL-3 (8)sulfurisotopeswereadoptedinthelatestJEF2.2crosssection (9)compilations.TheENDF/B-VIlibrary (3)doesnotincludetheindividualsulfurisotopic
cross sections.
in a drying oven for 24 h at 150°C. Place the dried Li SO in are several isotopes that can be used as reference standards for
2 4
a dessicator for cooling and storage. Prepare a phosphorus this monitoring. One is Pa, having a maximum beta energy
carrier solution by dissolving 21.3 g of (NH ) HPO in water of about 2000 keV, comparable to the 1710-keVbeta from P.
4 2 4
to make 1 L of solution. Prepare a Li SO sample for It is obtained as a daughter of U, that can be dispersed as a
2 4
irradiation by placing about 150 mg of material in an air-tight powder in plastic granules and formed to the shape of a
aluminum capsule or other suitable container. Following the standard pellet. The concentration of U can be varied to
irradiation, accurately weigh a sample of about 100 mg and obtain the desired counting rate. Uranium alpha particles can
dissolve in 5 mL of phosphorus carrier solution to minimize be prevented from reaching the detector by use of a 7-mg/cm
32 210
adsorptionof Pontheglasscontainer.Adropofconcentrated absorber. Another useful isotope is Bi that produces beta
HCl may be used to speed solution of the sample. Place the particleshavingamaximumenergyof1161keV.Itisobtained
solution in a volumetric flask and add additional phosphorus as a daughter of Pb, and sources are commercially avail-
carrier solution to bring the total volume to 100 mL. Prepare a able.
sample for counting by pipetting 0.050 mL of the P solution
10. Activity and Fluence by Detector Efficiency Method
onto a standard planchet and evaporating in air to dryness.
Counting procedures and calculations are the same as in other 10.1 Using a sulfur sample irradiated in a calibration neu-
methods with the exception that an aliquot factor of 2000 must tron field, determine the efficiency, ε, for the detector system:
be introduced for the 0.050-mL sample removed from the
Cf exp@λt # λ t
τ d i
ε 5 (1)
100-mL flask.
N σ¯ Φ 1 2 exp 2λt 1 2 exp 2λt
~ @ #!~ @ #!
s c i
9. Calibration where:
C = counts recorded in detector, less background,
9.1 Calibration is achieved by irradiation of sulfur in a
f = correction for coincidence losses, if needed,
fast-neutron field of known spectrum and intensity, and mea- τ
32 −7 −1
λ = P decay constant,=5.625×10 s ,
suring the resulting P activity to determine a counting
t = decay time, s,
d
system’s efficiency. This calibration is specific for a given
t = count time, s,
c
detector system, counting geometry, and sulfur pellet size and
t = duration of irradiation, s,
i
mass or sample preparation. It is, however, valid for subse-
N = number of S atoms in pellet,
quentuseinmeasuringactivitiesinanyarbitraryspectrum,and
σ¯ = spectrum-averaged cross section for S in the calibra-
s
therefore, may be used with activation data from other foils in 2 24
tion neutron field, cm =10 b, and
determining neutron energy spectra as described in Practice 2
Φ = neutron fluence, n/cm .
E721 and Practice E944.
10.1.1 Fig. 1 shows a plot of sulfur cross section as a
235 252
9.2 U fission and Cf spontaneous fission neutron
functionofenergy.Fig.2showsaplotoftheuncertaintyinthe
sources of known source strength are available for direct
sulfur cross section as a function of energy. (See also Guide
free-field calibrations (10). 252
E1018.) The spectrum-averaged cross section for Cf fission
9.3 Once a sulfur counting system is calibrated, it must be neutrons is about 70 mb, and for U fission is about 63 mb.
monitored t
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.