ASTM F2503-05
(Practice)Standard Practice for Marking Medical Devices and Other Items for Safety in the Magnetic Resonance Environment
Standard Practice for Marking Medical Devices and Other Items for Safety in the Magnetic Resonance Environment
SIGNIFICANCE AND USE
Medical devices and other items have caused serious injuries and death for patients and other individuals in the MR environment.
This practice provides a uniform system for marking to indicate the MR conditions that have been determined to be acceptable for a medical device or other item. It provides simple visual icons and terms which are intended to reduce injuries and other mishaps that occur when items that pose hazards in the MR environment are brought into the MR environment.
SCOPE
1.1 This practice covers the marking of medical devices and other items to indicate their safety in the magnetic resonance (MR) environment.
1.2 The purpose of this practice is to (1) recommend that items that may be brought into the MR environment be permanently marked to indicate the MR environment to which a specific item may safely be exposed, and (2) recommend information that should be included in the marking. It is recognized that direct marking on the item is not practical for implants and certain other medical devices. Where direct marking is not practical, this practice recommends that the marking be included in the labeling and on patient information cards (see 7.1).
1.3 Image artifact is not considered to be a safety issue and so is not addressed in this practice (see X1.5).
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
Designation:F2503–05
Standard Practice for
Marking Medical Devices and Other Items for Safety in the
Magnetic Resonance Environment
This standard is issued under the fixed designation F 2503; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope duced Torque on Medical Devices in the Magnetic Reso-
nance Environment
1.1 This practice covers the marking of medical devices and
2.2 Other Standards:
other items to indicate their safety in the magnetic resonance
ISO 3864-1:2002(E) Graphical Symbols—Safety Colours
(MR) environment.
and Safety Signs—Part 1: Design Principles for Safety
1.2 The purpose of this practice is to (1) recommend that
Signs in Workplaces and Public Areas
items that may be brought into the MR environment be
ISO 13485:2003(E) Medical Devices—Quality Manage-
permanently marked to indicate the MR environment to which
ment Systems—Requirements for Regulatory Purposes,
a specific item may safely be exposed, and (2) recommend
definition 3.7
information that should be included in the marking. It is
ISO/IEC Guide 51:1999, definition 3.5
recognized that direct marking on the item is not practical for
IEC 60601-2-33, Ed. 2.0 Medical Electrical Equipment—
implants and certain other medical devices. Where direct
Part2:ParticularRequirementsfortheSafetyofMagnetic
marking is not practical, this practice recommends that the
Resonance Equipment for Medical Diagnosis
marking be included in the labeling and on patient information
cards (see 7.1).
3. Terminology
1.3 Image artifact is not considered to be a safety issue and
3.1 Definitions:
so is not addressed in this practice (see X1.5).
3.1.1 hazard—potential source of harm. ISO/IEC
1.4 This standard does not purport to address all of the
Guide 51
safety concerns, if any, associated with its use. It is the
3.1.2 item—medical device or other object that may be
responsibility of the user of this standard to establish appro-
brought into the MR environment.
priate safety and health practices and determine the applica-
3.1.3 magnetically induced displacement force—force pro-
bility of regulatory limitations prior to use.
ducedwhenamagneticobjectisexposedtothespatialgradient
2. Referenced Documents of a static magnetic field. This force will tend to cause the
2 object to translate in the spatial gradient of the static magnetic
2.1 ASTM Standards:
field.
F 2052 Test Method for Measurement of Magnetically In-
3.1.4 magnetically induced torque—torque produced when
duced Displacement Force on Medical Devices in the
a magnetic object is exposed to a magnetic field. This torque
Magnetic Resonance Environment
will tend to cause the object to align itself along the magnetic
F2119 Test Method for Evaluation of MR Image Artifacts
field in an equilibrium direction that induces no torque.
from Passive Implants
3.1.5 magnetic induction or magnetic flux density (B in
F 2182 Test Method for Measurement of Radio Frequency
T)—that magnetic vector quantity which at any point in a
Induced Heating Near Passive Implants During Magnetic
magnetic field is measured either by the mechanical force
Resonance Imaging
experiencedbyanelementofelectriccurrentatthepoint,orby
F 2213 Test Method for Measurement of Magnetically In-
the electromotive force induced in an elementary loop during
any change in flux linkages with the loop at the point. The
magnetic induction is frequently referred to as the magnetic
ThispracticeisunderthejurisdictionofASTMCommitteeF04onMedicaland
Surgical Materials and Devices and is the direct responsibility of Subcommittee
F04.15 on Material Test Methods.
Current edition approved Aug. 1, 2005. Published August 2005.
2 3
For referenced ASTM standards, visit the ASTM website, www.astm.org, or Available fromAmerican National Standards Institute (ANSI), 25 W. 43rd St.,
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM 4th Floor, New York, NY 10036.
Standards volume information, refer to the standard’s Document Summary page on Available from International Electrotechnical Commission (IEC), 3 rue de
the ASTM website. Varembé, Case postale 131, CH-1211, Geneva 20, Switzerland.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
NOTICE: This standard has either been superseded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
F2503–05
field. B isthestaticfieldinanMRsystem.Plaintypeindicates constant, 42.56 MHz/T for protons, and B is the static
0 0
a scalar (for example, B) and bold type indicates a vector (for magnetic field in Tesla.
example,B). 3.1.14 safety—freedom from unacceptable risk in the MR
3.1.6 magnetic resonance (MR)—resonant absorption of environment.
3.1.15 specific absorption rate (SAR)—themassnormalized
electromagnetic energy by an ensemble of atomic particle
situated in a magnetic field. rate at which RF energy is deposited in biological tissue. SAR
is typically indicated in W/kg.
3.1.7 magnetic resonance (MR) environment—volume
within the 0.50 mT (5 gauss (G)) line of an MR system, which 3.1.16 tesla, (T)—the SI unit of magnetic induction equal to
10 gauss (G).
includes the entire three dimensional volume of space sur-
rounding the MR scanner. For cases where the 0.50 mT line is
4. Significance and Use
contained within the Faraday shielded volume, the entire room
4.1 Medical devices and other items have caused serious
shall be considered the MR environment.
injuries and death for patients and other individuals in the MR
3.1.8 magnetic resonance system (MR system)—ensemble
environment.
of MR equipment, accessories, including means for display,
4.2 This practice provides a uniform system for marking to
control, energy supplies, and the MR environment.
indicate the MR conditions that have been determined to be
IEC 60601-2-33
acceptable for a medical device or other item. It provides
3.1.9 MR Conditional—an item that has been demonstrated
simple visual icons and terms which are intended to reduce
to pose no known hazards in a specified MR environment with
injuries and other mishaps that occur when items that pose
specified conditions of use. Field conditions that define the
hazards in the MR environment are brought into the MR
specified MR environment include field strength, spatial gra-
environment.
dient, dB/dt (time rate of change of the magnetic field), radio
frequency (RF) fields, and specific absorption rate (SAR).
5. Methods of Marking
Additional conditions, including specific configurations of the
5.1 The marking method should not compromise perfor-
item, may be required.
mance or function of the marked item and should provide
3.1.10 MR Safe—anitemthatposesnoknownhazardsinall
legibility over the anticipated service life of the item.
MR environments.
6. Required Information
NOTE 1—MR Safe items include nonconducting, nonmagnetic items
such as a plastic Petri dish.An item may be determined to be MR Safe by
6.1 Perform testing sufficient to characterize the behavior of
providing a scientifically based rationale rather than test data.
the item in the MR environment. In particular, testing for items
that may be placed in the MR environment should address
3.1.11 MR Unsafe—an item that is known to pose hazards
magnetically induced displacement force (Test Method
in all MR environments.
F 2052), magnetically induced torque (Test Method F 2213),
NOTE 2—MR Unsafe items include magnetic items such as a pair of
and RF heating (Test Method F 2182).
ferromagnetic scissors.
NOTE 3—Other possible safety issues include but are not limited to,
3.1.12 medical device—any instrument, apparatus, imple-
thermal injury, induced currents/voltages, electromagnetic compatibility,
ment, machine, appliance, implant, in vitro reagent or calibra-
neurostimulation, acoustic noise, interaction among devices, and the safe
tor, software, material, or other similar or related article,
functioning of the item and the safe operation of the MR system.
intended by the manufacturer to be used, alone or in combi-
6.2 Any parameter that affects the safety of the item should
nation, for human beings for one or more of the specific
be listed. Any condition that is known to produce an unsafe
purpose(s) of:
condition must be described.
(1) diagnosis, prevention, monitoring, treatment, or allevia-
tion of disease, 7. Information Included in MR Marking
(2) diagnosis, monitoring, treatment, alleviation of, or com-
7.1 Medicaldevicesandotheritemsvarywidelyinsize,and
pensation for an injury,
the amount of information that practically can be included in
(3) investigation, replacement, modification, or support of
marking varies accordingly. For implants, the MR marking
the anatomy or of a physiological process,
should be included in the package labeling (including the
(4) supporting or sustaining life,
instructions for use and package inserts) and on the patient
(5) control of conception,
information card. For nonimplanted items intended to be used
(6) disinfection of medical devices,
in the MR environment, the MR marking should be positioned
(7) providing information for medical purposes by means of in a prominent location on the item as well as in the item
in vitro examination of specimens derived from the human
labeling.Someitems(forexample,smallorverythinitems)do
body, and which does not achieve its primary intended action not provide any surfaces which can be marked practically. For
in or on the human body by pharmacological, immunological,
items for which direct marking is not practical, the MR
or metabolic means, but which may be assisted in its function marking should be included in the labeling.
by such means. ISO 13485
7.2 Minimum Information—As a result of the testing de-
3.1.13 radio frequency (RF) magnetic field—the magnetic scribed in Section 6, mark the item as MR Safe, MR Condi-
field in MRI that is used to flip the magnetic moments. The tional, or MR Unsafe using the icons as shown in Tables 1 and
frequency of the RF field is gB where g is the gyromagnetic 2.
NOTICE: This standard has either been superseded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
F2503–05
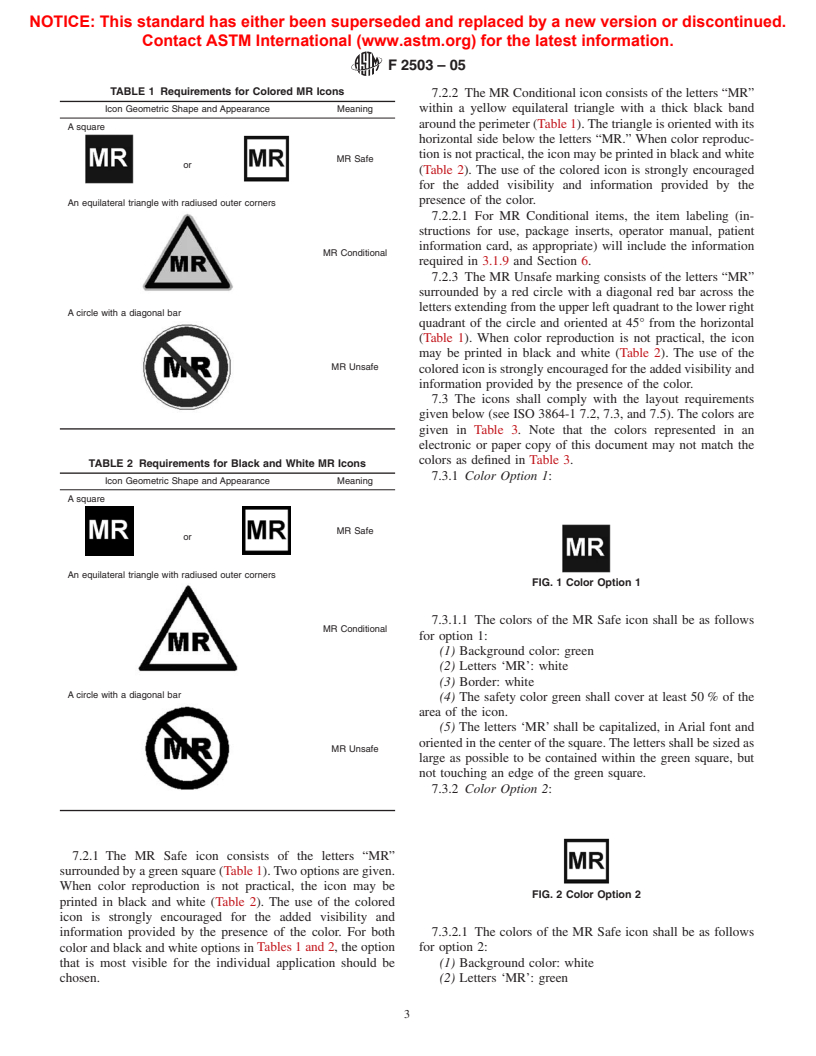
TABLE 1 Requirements for Colored MR Icons
7.2.2 The MR Conditional icon consists of the letters “MR”
Icon Geometric Shape and Appearance Meaning within a yellow equilateral triangle with a thick black band
around the perimeter (Table 1).The triangle is oriented with its
A square
horizontal side below the letters “MR.” When color reproduc-
tion is not practical, the icon may be printed in black and white
MR Safe
or
(Table 2). The use of the colored icon is strongly encouraged
for the added visibility and information provided by the
presence of the color.
An equilateral triangle with radiused outer corners
7.2.2.1 For MR Conditional items, the item labeling (in-
structions for use, package inserts, operator manual, patient
information card, as appropriate) will include the information
MR Conditional
required in 3.1.9 and Section 6.
7.2.3 The MR Unsafe marking consists of the letters “MR”
surrounded by a red circle with a diagonal red bar across the
lettersextendingfromtheupperleftquadranttothelowerright
A circle with a diagonal bar
quadrant of the circle and oriented at 45° from the horizontal
(Table 1). When color reproduction is not practical, the icon
may be printed in black and white (Table 2). The use of the
MR Unsafe
colorediconisstronglyencouragedfortheaddedvisibilityand
information provided by the presence of the color.
7.3 The icons shall comply with the layout requirements
given below (see ISO 3864-1 7.2, 7.3, and 7.5). The colors are
given in Table 3. Note that the colors represented in an
electronic or paper copy of this document may not match the
colors as defined in Table 3.
TABLE 2 Requirements for Black and White MR Icons
7.3.1 Color Option 1:
Icon Geometric Shape and Appearance Meaning
A square
MR Safe
or
An equilateral triangle with radiused outer corners
FIG. 1 Color Option 1
7.3.1.1 The colors of the MR Safe icon shall be as follows
MR Conditional
for option 1:
(1) Background color: green
(2) Letters ‘MR’: white
(3) Border: white
A circle with a diagonal bar
(4) The safety color green shall cover at least 50 % of the
area of the icon.
(5) The letters ‘MR’shall be capitalized, in Arial font and
orientedinthecenterofthesquare.Thelettersshallbesizedas
MR Unsafe
large as possible to be contained within the green square, but
not touching an edge of the green square.
7.3.2 Color Option 2:
7.2.1 The MR Safe icon consists of the letters “MR”
surrounded by a green square (Table 1).Two options are given.
When color reproduction is not practical, the icon may be
FIG. 2 Color Option 2
printed in black and white (Table 2). The use of the colored
icon is strongly encouraged for the added visibility and
information provided by the presence of the color. For both 7.3.2.1 The colors of the MR Safe icon shall be as follows
color and black and white options inTables 1 and 2, the option for option 2:
that is most visible for the individual application should be (1) Background color: white
chosen. (2) Letters ‘MR’: green
NOTICE: This standard has either been superseded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
F2503–05
TABLE 3 Examples from Color Order Systems for the Icon Colors
A
(DIN, RAL, Munsell, AFNOR, and NCS examples from ISO3864–1:2)
AFNOR
DIN 5381
Color RAL Munsell NF X08-002 NCS Pantone
DIN 6164
and X08-010
Red 7,5 : 8,5 :3 RAL 3001 7,5R 4/14 N°2805 S 2080-R Pantone 1807 C
Yellow 2,5 : 6,5 : 1 RAL 1003 10YR 7/14 N°1330 S 1070-Y10R Pantone 1235 C
Green 21,7 : 6,5 : 4 RAL 6032 5G 4/9 N°2455 S 3060-G Pantone 3415 C
White N : 0 : 0,5 RAL 9003 N 9,5 N°3665 S 0500-N Pantone White
Black N : 0 :9 RAL 9004 N 1 N°2603 S 9000-N Pantone 6 C
A
RInternationalOrganizationforStandardization(ISO).ThismaterialisreproducedfromISO3864–1:2002withpermissionoftheAmericanNationalStandardsInstitute
on behalf of ISO. No part of this material may be copied or reproduced in any form, electronic retrieval system or otherwise or made available on the Internet, a public
network, by satellite or otherwise without prior written consent of theAmerican National Standards Institute (ANSI), 25 West 43rd Street, New York, NY 10036. Copies of
this standard may be purchased from the ANSI, (212)
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.