ASTM F2978-20
(Guide)Standards Guide to Optimize Scan Sequences for Clinical Diagnostic Evaluation of Metal-on-Metal Hip Arthroplasty Devices using Magnetic Resonance Imaging
Standards Guide to Optimize Scan Sequences for Clinical Diagnostic Evaluation of Metal-on-Metal Hip Arthroplasty Devices using Magnetic Resonance Imaging
SIGNIFICANCE AND USE
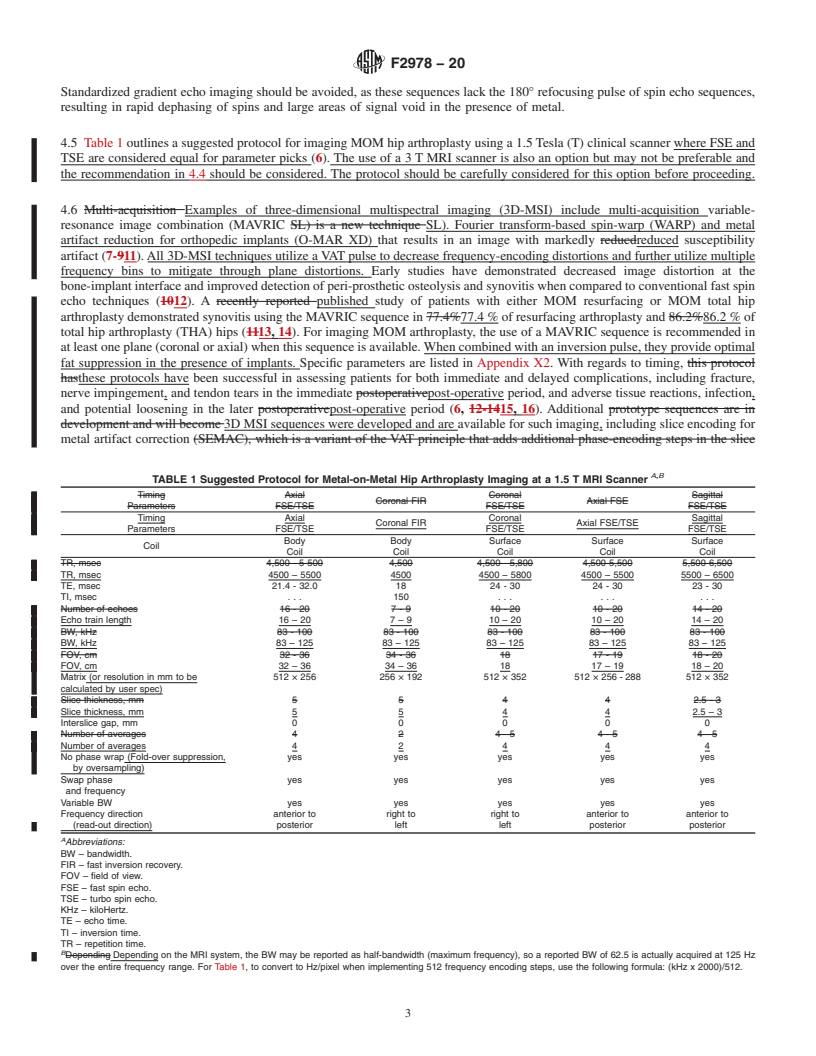
5.1 Magnetic resonance imaging is ideally suited to image MOM hip arthroplasty due to its superior soft tissue contrast, multiplanar capabilities and lack of ionizing radiation. MR imaging is the most accurate imaging modality for the assessment of peri-prosthetic osteolysis and wear-induced synovitis (19, 20).
5.2 Before scanning a patient with a specific implant, the MR practitioner shall confirm that the device is MR Conditional and that the scan protocol to be used satisfies the conditions for safe scanning for the specific implant.
5.3 This guide can be used to identify the following adverse events.
5.3.1 Osteolysis—Magnetic resonance imaging is superior to conventional radiographs and computer tomography (CT) in the assessment of peri-prosthetic osteolysis and has been shown to be the most accurate method to locate and quantify the extent of peri-prosthetic osteolysis (19, 21). On MR imaging, osteolysis appears as well marginated intraosseous intermediate to slightly increased signal intensity lesions that contrast with the high signal intensity of the intramedullary fat. A characteristic line of low signal intensity surrounds the area of focal marrow replacement, distinguishing the appearance of osteolysis from tumoral replacement of bone or infection (22).
FIG. 4 Coronal (left) and Axial (right) FSE Images of a Left MOM Hip Arthroplasty
Note 1: There is focal osteolysis (white arrows) in the greater trochanter, which manifests as well-demarcated intermediate signal intensity, similar to that of skeletal muscle, replacing the normal high signal intensity fatty marrow. Images courtesy of Dr. Hollis Potter.
5.3.2 Component Loosening—While the data are preliminary, MR imaging can identify circumferential bone resorption that may indicate component loosening. Loosening may result from osteolysis, circumferential fibrous membrane formation or poor osseous integration of a non-cemented component. On MR imaging, component loosening typically manif...
SCOPE
1.1 This guide describes the recommended protocol for magnetic resonance imaging (MRI) studies of patients implanted with metal-on-metal (MOM) devices to determine if the periprosthetic tissues are likely to be associated with an adverse local tissue reaction (ALTR). Before scanning a patient with a specific implant, the MR practitioner shall confirm that the device is MR Conditional and that the scan protocol to be used satisfies the conditions for safe scanning for the specific implant. This guide assumes that the MRI protocol will be applied to MOM devices while they are implanted inside the body. It is also expected that standardized MRI safety measures will be followed during the performance of this scan protocol.
1.2 This guide covers the clinical evaluation of the tissues surrounding MOM hip replacement devices in patients using MRI. This guide is applicable to both total and resurfacing MOM hip systems.
1.3 The protocol contained in this guide applies to whole body magnetic resonance equipment, as defined in section 201.3.239 of IEC 60601-2-33, Ed. 3.2, with a whole body radiofrequency (RF) transmit coil as defined in section 201.3.240. The RF coil should have circulary polarized RF excitation (also commonly referred to as quadrature excitation) as defined in section 201.3.249 of IEC 60601-2-33, Ed. 3.2..
1.4 The values stated in SI units are to be regarded as standard.
1.5 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of regulatory limitations prior to use. The user may consider all precautions and warnings provided in the MR system and hip implant labeling prior to determining the applicability of these protocols.
1.6 This international standard was developed in accordance with internatio...
General Information
Buy Standard
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
Designation: F2978 − 20
Standard Guide to
Optimize Scan Sequences for Clinical Diagnostic Evaluation
of Metal-on-Metal Hip Arthroplasty Devices using Magnetic
1
Resonance Imaging
This standard is issued under the fixed designation F2978; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope in the MR system and hip implant labeling prior to determining
the applicability of these protocols.
1.1 This guide describes the recommended protocol for
1.6 This international standard was developed in accor-
magnetic resonance imaging (MRI) studies of patients im-
dance with internationally recognized principles on standard-
planted with metal-on-metal (MOM) devices to determine if
ization established in the Decision on Principles for the
the periprosthetic tissues are likely to be associated with an
Development of International Standards, Guides and Recom-
adverselocaltissuereaction(ALTR).Beforescanningapatient
mendations issued by the World Trade Organization Technical
with a specific implant, the MR practitioner shall confirm that
Barriers to Trade (TBT) Committee.
the device is MR Conditional and that the scan protocol to be
used satisfies the conditions for safe scanning for the specific
2. Referenced Documents
implant. This guide assumes that the MRI protocol will be
2
2.1 ASTM Standards:
applied to MOM devices while they are implanted inside the
A340 Terminology of Symbols and Definitions Relating to
body.ItisalsoexpectedthatstandardizedMRIsafetymeasures
Magnetic Testing
will be followed during the performance of this scan protocol.
F2503 Practice for Marking Medical Devices and Other
1.2 This guide covers the clinical evaluation of the tissues
Items for Safety in the Magnetic Resonance Environment
3
surrounding MOM hip replacement devices in patients using
2.2 IEC Standard:
MRI. This guide is applicable to both total and resurfacing
IEC 60601-2-33:2010+AMD1:2013+AMD2:2015
MOM hip systems.
CSV Medical electrical equipment—Part 2: Particular re-
quirements for the basic safety and essential performance
1.3 The protocol contained in this guide applies to whole
of magnetic resonance equipment for medical diagnosis,
body magnetic resonance equipment, as defined in section
2015
201.3.239 of IEC 60601-2-33, Ed. 3.2, with a whole body
radiofrequency (RF) transmit coil as defined in section
3. Terminology
201.3.240. The RF coil should have circulary polarized RF
3.1 Definitions—For the purposes of this standard the
excitation (also commonly referred to as quadrature excitation)
following definitions shall apply:
as defined in section 201.3.249 of IEC 60601-2-33, Ed. 3.2.
3.1.1 Magnetic Resonance Imaging (MRI)—diagnostic im-
1.4 The values stated in SI units are to be regarded as
aging technique that uses static and time-varying magnetic
standard.
fields to provide tomographic images of tissue by the magnetic
resonance of nuclei.
1.5 This standard does not purport to address all of the
safety concerns, if any, associated with its use. It is the 3.1.2 MR-Conditional—an item with demonstrated safety in
responsibility of the user of this standard to establish appro-
theMRenvironmentwithindefinedconditions.Ataminimum,
priate safety, health, and environmental practices and deter-
address the conditions of the static magnetic field, the switched
mine the applicability of regulatory limitations prior to use.
gradient magnetic field and the radiofrequency fields. Addi-
The user may consider all precautions and warnings provided
tional conditions, including specific configurations of the item,
may be required (Practice F2503 – 13).
1 2
This guide is under the jurisdiction of ASTM Committee F04 on Medical and For referenced ASTM standards, visit the ASTM website, www.astm.org, or
Surgical Materials and Devices and is the direct responsibility of Subcommittee contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
F04.22 on Arthroplasty. Standards volume information, refer to the standard’s Document Summary page on
Current edition approved Sept. 1, 2020. Published October 2020. Originally the ASTM website.
3
approved in 2013. Last previous edition approved in 2013 as F2978 – 13. DOI: Available from International Electrotechnical Commission (IEC), 3, rue de
10.1520/F2978-20. Varembé, P.O. Box 131, CH-1211 Geneva 20, Switzerland, http://www.iec.ch.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
...
This document is not an ASTM standard and is intended only to provide the user of an ASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
Designation: F2978 − 13 F2978 − 20
Standards Guide to
Optimize Scan Sequences for Clinical Diagnostic Evaluation
of Metal-on-Metal Hip Arthroplasty Devices using Magnetic
1
Resonance Imaging
This standard is issued under the fixed designation F2978; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope
1.1 This guide describes the recommended protocol for magnetic resonance imaging (MRI) studies of patients implanted with
metal-on-metal (MOM) devices to determine if the periprosthetic tissues are likely to be associated with an adverse local tissue
reaction (ALTR). Before scanning a patient with a specific implant, the MR practitioner shall confirm that the device is MR
Conditional and that the scan protocol to be used satisfies the conditions for safe scanning for the specific implant. This guide
assumes that the MRI protocol will be applied to MOM devices while they are implanted inside the body. It is also expected that
standardized MRI safety measures will be followed during the performance of this scan protocol.
1.2 This guide covers the clinical evaluation of the tissues surrounding MOM hip replacement devices in patients using MRI. This
guide is applicable to both total and resurfacing MOM hip systems.
1.3 The protocol contained in this guide applies to whole body magnetic resonance equipment, as defined in section
2.2.103201.3.239 of IEC 60601-2-33, Ed. 3.0,3.2, with a whole body radiofrequency (RF) transmit coil as defined in section
2.2.100.201.3.240. The RF coil should have quadrature excitation.circulary polarized RF excitation (also commonly referred to as
quadrature excitation) as defined in section 201.3.249 of IEC 60601-2-33, Ed. 3.2.
1.4 The values stated in SI units are to be regarded as standard.
1.5 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of
the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory
limitations prior to use. The user may consider all precautions and warnings provided in the MR system and hip implant labeling
prior to determining the applicability of these protocols.
1.5 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility
of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of
regulatory limitations prior to use. The user may consider all precautions and warnings provided in the MR system and hip implant
labeling prior to determining the applicability of these protocols.
1.6 This international standard was developed in accordance with internationally recognized principles on standardization
established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued
by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
1
This guide is under the jurisdiction of ASTM Committee F04 on Medical and Surgical Materials and Devices and is the direct responsibility of Subcommittee F04.22
on Arthroplasty.
Current edition approved Dec. 1, 2013Sept. 1, 2020. Published May 2014October 2020. Originally approved in 2013. Last previous edition approved in 2013 as
F2978 – 13. DOI: 10.1520/F2978-13.10.1520/F2978-20.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
F2978 − 20
2. Referenced Documents
2
2.1 ASTM Standards:
A340 Terminology of Symbols and Definitions Relating to Magnetic Testing
F2503 Practice for Marking Medical Devices and Other Items for Safety in the Magnetic Resonance Environment
3
2.2 IEC Standard:
IEC 60601-2-33, Ed. 3.0IEC 60601-2-33:2010+AMD1:2013+AMD2:2015 CSV Medical Electrical Equipment—Partelectrical
equipment—Part 2: Particular Requirementsrequirements for the Safety of Magnetic Resonance Equipment for Medical
Diagnosis, 2010basic safety and essential performance of magnetic resonance equipment for medical diagnosis, 2015
3. Terminology
3.1 Definitions—For the purposes of this standard the following definitions shall apply:
3.1.1 Magnetic Resonance Imaging (MRI)—diagnostic imaging technique t
...










Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.