ASTM E1269-99
(Test Method)Standard Test Method for Determining Specific Heat Capacity by Differential Scanning Calorimetry
Standard Test Method for Determining Specific Heat Capacity by Differential Scanning Calorimetry
SCOPE
1.1 This test method covers the determination of specific heat capacity by differential scanning calorimetry.
1.2 This test method is generally applicable to thermally stable solids and liquids.
1.3 The normal operating range of the test is from 100 to 600oC. The temperature range can be extended, depending upon the instrumentation and specimen holders used.
1.4 The values stated in SI units are to be regarded as the standard.
1.5 Computer or electronic-based instrumentation, techniques, or data treatment equivalent to this test method may be used.
Note 1--Users of this test method are expressly advised that all such instruments or techniques may not be equivalent. It is the responsibility of the user of this test method to determine equivalency prior to use.
1.6 This method is similar to ISO 11357-4, but contains additional methodology not found in that method. Additionally, ISO 11357-4 contains practices not found in this standard. This method is similar to Japanese Industrial Standard K 7123, but contains additional methodology not found in that method.
1.7 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use. Specific precautionary statements are given in Section 9.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
Designation: E 1269 – 99
Standard Test Method for
Determining Specific Heat Capacity by Differential Scanning
Calorimetry
This standard is issued under the fixed designation E 1269; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope 4. Summary of Test Method
1.1 This test method covers the determination of specific 4.1 This test method consists of heating the test material at
heat capacity by differential scanning calorimetry. a controlled rate in a controlled atmosphere through the region
1.2 This test method is generally applicable to thermally of interest. The difference in heat flow into the test material and
stable solids and liquids. a reference material or blank due to energy changes in the
1.3 The normal operating range of the test is from − 100 to material is continually monitored and recorded.
600°C. The temperature range can be extended, depending
5. Significance and Use
upon the instrumentation and specimen holders used.
5.1 Differential scanning calorimetric measurements pro-
1.4 The values stated in SI units are to be regarded as the
standard. vide a rapid, simple method for determining specific heat
capacities of materials.
1.5 Computer or electronic-based instrumentation, tech-
niques, or data treatment equivalent to this test method may be 5.2 Specific heat capacities are important for reactor and
cooling system design purposes, quality control, and research
used.
and development.
NOTE 1—Users of this test method are expressly advised that all such
instruments or techniques may not be equivalent. It is the responsibility of
6. Interferences
the user of this test method to determine equivalency prior to use.
6.1 Since milligram quantities of specimen are used, it is
1.6 This standard does not purport to address all of the
essential that specimens are homogeneous and representative.
safety concerns, if any, associated with its use. It is the
6.2 The occurrence of chemical changes or mass loss on
responsibility of the user of this standard to establish appro-
heating during the measurement may invalidate the test.
priate safety and health practices and determine the applica-
Therefore, the temperature range and specimen holders should
bility of regulatory limitations prior to use. Specific precau-
be chosen so as to avoid these processes.
tionary statements are given in Section 9.
7. Apparatus
2. Referenced Documents
7.1 Differential Scanning Calorimeter (DSC)—The essen-
2.1 ASTM Standards:
tial instrumentation required to provide the minimum differen-
E 473 Terminology Relating to Thermal Analysis
tial scanning calorimetric capability for this method includes:
E 967 Practice for Temperature Calibration of Differential
7.1.1 DSC Test Chamber, composed of the following:
Scanning Calorimeters and Differential Thermal Analyz-
7.1.1.1 Furnace(s), to provide uniform controlled heating
ers
(cooling) of a specimen and reference to a constant temperature
E 968 Practice for Heat Flow Calibration of Differential
or at a constant rate within the applicable –100 to 600 °C
Scanning Calorimeters and Differential Thermal Analyz-
temperature range of this test method.
ers
7.1.1.2 Temperature Sensor, to provide an indication of the
E 1142 Terminology Relating to Thermophysical Proper-
specimen temperature to 6 10 mK (0.01 °C).
ties
7.1.1.3 Differential Sensor, to detect heat flow difference
between the specimen and reference equivalent to 1 μW.
3. Terminology
7.1.1.4 A means of sustaining a test chamber environment
3.1 Definitions—Technical terms used in this test method
of inert purge gas at a purge flow rate of 10 to 50 mL/min 6
are described in Terminologies E 473 and E 1142.
5 mL/min.
NOTE 2—Typically, 99+ % pure nitrogen, argon, or helium are em-
This test method is under the jurisdiction of ASTM Committee E-37 on
ployed when oxidation in air is a concern. Unless effects of moisture are
Thermal Measurements and is the direct responsibility of Subcommittee E37.01 on
Test Methods and Recommended Practices.
to be studied, use of dry purge gas is recommended and is essential for
Current edition approved March 10, 1999. Published May 1999. Originally
operation at subambient temperatures.
published as E 1269–90. Last previous edition E 1269–95.
Annual Book of ASTM Standards, Vol 14.02.
Copyright © ASTM, 100 Barr Harbor Drive, West Conshohocken, PA 19428-2959, United States.
E 1269
7.1.2 Temperature Controller, capable of executing a spe- 11. Calibration
cific temperature program by operating the furnace(s) between
11.1 Specific heat capacity is a quantitative measurement of
selected temperature limits at a rate of temperature change of
energy made as a function of temperature. Thus, the instrument
10 to 20 °C/min constant to 6 0.1 °C/min or at an isothermal
used in its measurement must be calibrated in both the
temperature constant to 6 0.1 °C.
temperature and heat flow modes. Since specific heat capacity
7.1.3 Recording Device, either digital or analog, capable of
is not a rapidly changing function of temperature, the instru-
recording and displaying any fraction of the heat flow signal
ment’s temperature mode is ordinarily calibrated and checked
(DSC curve) including the signal noise as a function of
only occasionally. The heat flow information, however, is
temperature.
much more critical and becomes an integral part of the specific
7.1.4 While not required, the user may find useful software
heat capacity measurement through the use of a reference
to perform the mathematical treatments described in this test
material.
method.
11.2 Perform any calibration procedures described by the
7.1.5 Containers (pans, crucibles, vials, etc., and lids) that
manufacturer in the operations manual.
are inert to the specimen and reference materials and which are
11.3 Perform a temperature calibration for the apparatus
of suitable structural shape and integrity to contain the speci-
using Practice E 967.
men and reference in accordance with the specific requirements
11.4 Perform a heat flow calibration for the apparatus using
of this test method.
Practice E 968.
7.1.6 Cooling capability to hasten cool down from elevated
11.5 Heat Flow Calibration:
temperatures, to provide constant cooling rates of up to 10
11.5.1 Synthetic sapphire disk (a-aluminum oxide; alu-
°C/min, to achieve subambient operation, or to sustain an
mina) is recommended as a heat flow calibration standard for
isothermal subambient temperature, or a combination thereof.
specific heat capacity measurements. Specific heat capacity
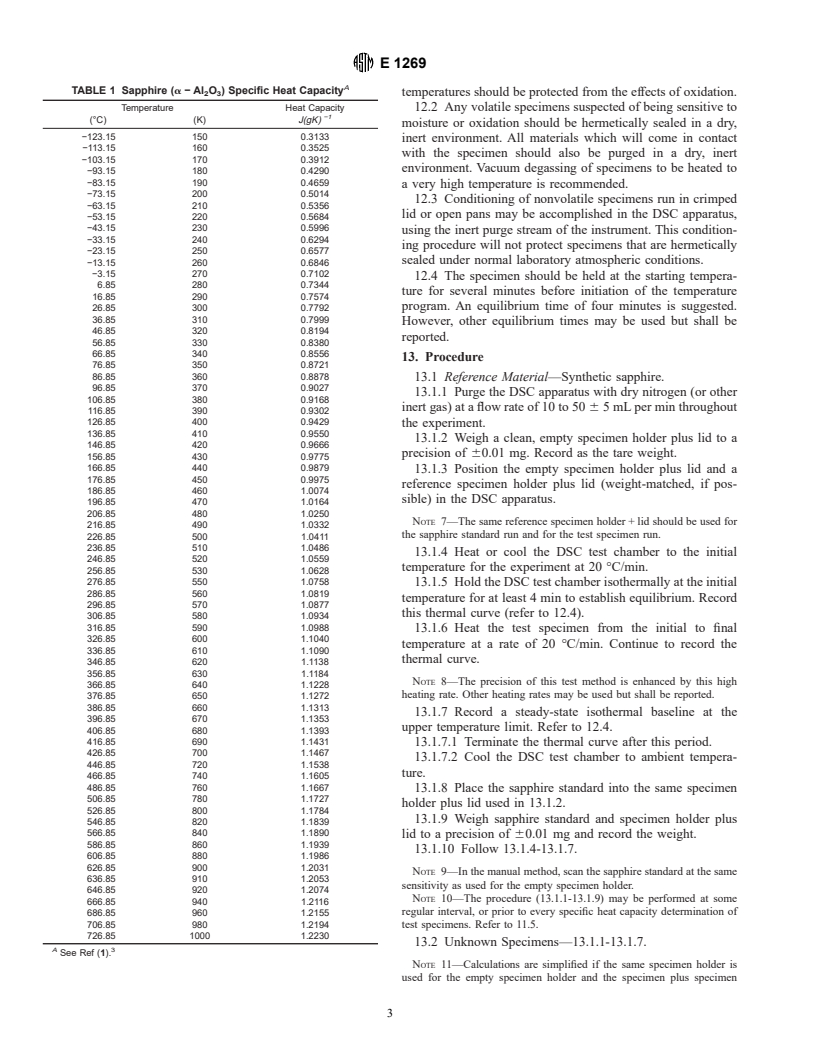
7.2 Balance, with a capacity of 100 mg or greater to weigh
values for synthetic sapphire are given in Table 1.
specimens or containers, or both, to6 10 μg.
NOTE 5—It is possible to use other standard materials or other physical
8. Reagents and Materials
forms of synthetic sapphire, but their use should be noted in the report.
The potential adverse impact of increased interfacial resistance encoun-
8.1 Specific heat capacity standard: synthetic sapphire disk,
tered with granular/textured samples may be minimized with the use of a
10 to 100 mg.
powdered synthetic sapphire standard. It is preferred that the physical
NOTE 3—Interlaboratory studies indicate that physical forms of the form of the sample be similar to that of the standard.
synthetic sapphire other than disks give lower precision and greater bias
11.5.2 The heat flow calibration may be performed at some
in the results.
regular interval or prior to every specific heat capacity deter-
9. Hazards mination or test specimens.
9.1 Safety Precautions—If a specimen is heated to decom-
NOTE 6—A frequency of calibration of at least once a day is recom-
position, toxic or corrosive products may be released. mended. Other time intervals may be selected for heat flow calibration but
should be noted in the report.
9.2 Technical Precautions:
9.2.1 The same heating rate should be used for both the
11.5.3 If the heat flow calibration is performed at a regular
calibration and specimen runs.
interval, the calorimetric sensitivity, E, may be calculated using
9.2.2 Precision of heating rate, placement of the specimen
the specific heat capacity values for synthetic sapphire given in
holder, use of flat specimen holders, and the establishment of
Table 1 and the following equation:
equilibrium are essential. Instrument settings should not be
E 5 b/ 60 · Dst! Wst · Cp st!1DW· Cp c! (1)
@ ~ #@ ~ ~ #
adjusted once a specific heat capacity calibration has been
Refer to Section 13 for the procedure and Section 14 for the
performed.
list of symbols.
10. Sampling
11.5.4 If the heat flow calibration is performed prior to
10.1 Powdered or granular specimens should be mixed prior
every specific heat capacity determination, it is unnecessary to
to sampling and should be sampled by removing portions from
calculate the calorimetric sensitivity, E. Refer to Section 13 for
various parts of the container. These portions, in turn, should be
the procedure.
combined and mixed to ensure a representative specimen for
12. Conditioning
the determinations.
10.2 Liquid specimens may be sampled directly after stir-
12.1 Specimens and specimen holders for specific heat
ring.
capacity determinations may be handled in ordinary laboratory
10.3 Solid specimens may be sampled by cutting or slicing
environments for screening or qualitative measurements. How-
with a clean knife or razor blade. Sample uniformity should be
ever, if quantitative data are needed over a wide temperature
ascertained, since segregation within the solid is possible.
range, specimen conditioning may be required. Specimens
which will be exposed to low temperatures should be protected
NOTE 4—Solid specimens should be so sampled as to maximize contact
from moisture. Specimens that will be exposed to very high
with the surface of the specimen holder.
10.4 Samples are usually analyzed as received. If some heat
or mechanical treatment is applied to the specimen prior to
The boldface numbers in parentheses refer to the list of references at the end of
analysis, this treatment should be noted in the report. this standard.
E 1269
A
TABLE 1 Sapphire (a−Al O ) Specific Heat Capacity
temperatures should be protected from the effects of oxidation.
2 3
Temperature Heat Capacity 12.2 Any volatile specimens suspected of being sensitive to
−1
(°C) (K) J(gK)
moisture or oxidation should be hermetically sealed in a dry,
−123.15 150 0.3133
inert environment. All materials which will come in contact
−113.15 160 0.3525
with the specimen should also be purged in a dry, inert
−103.15 170 0.3912
environment. Vacuum degassing of specimens to be heated to
−93.15 180 0.4290
−83.15 190 0.4659
a very high temperature is recommended.
−73.15 200 0.5014
12.3 Conditioning of nonvolatile specimens run in crimped
−63.15 210 0.5356
lid or open pans may be accomplished in the DSC apparatus,
−53.15 220 0.5684
−43.15 230 0.5996
using the inert purge stream of the instrument. This condition-
−33.15 240 0.6294
ing procedure will not protect specimens that are hermetically
−23.15 250 0.6577
sealed under normal laboratory atmospheric conditions.
−13.15 260 0.6846
−3.15 270 0.7102
12.4 The specimen should be held at the starting tempera-
6.85 280 0.7344
ture for several minutes before initiation of the temperature
16.85 290 0.7574
program. An equilibrium time of four minutes is suggested.
26.85 300 0.7792
36.85 310 0.7999
However, other equilibrium times may be used but shall be
46.85 320 0.8194
reported.
56.85 330 0.8380
66.85 340 0.8556
13. Procedure
76.85 350 0.8721
86.85 360 0.8878
13.1 Reference Material—Synthetic sapphire.
96.85 370 0.9027
13.1.1 Purge the DSC apparatus with dry nitrogen (or other
106.85 380 0.9168
inert gas) at a flow rate of 10 to 50 6 5 mL per min throughout
116.85 390 0.9302
126.85 400 0.9429 the experiment.
136.85 410 0.9550
13.1.2 Weigh a clean, empty specimen holder plus lid to a
146.85 420 0.9666
precision of 60.01 mg. Record as the tare weight.
156.85 430 0.9775
166.85 440 0.9879
13.1.3 Position the empty specimen holder plus lid and a
176.85 450 0.9975
reference specimen holder plus lid (weight-matched, if pos-
186.85 460 1.0074
sible) in the DSC apparatus.
196.85 470 1.0164
206.85 480 1.0250
NOTE 7—The same reference specimen holder + lid should be used for
216.85 490 1.0332
the sapphire standard run and for the test specimen run.
226.85 500 1.0411
236.85 510 1.0486
13.1.4 Heat or cool the DSC test chamber to the initial
246.85 520 1.0559
temperature for the experiment at 20 °C/min.
256.85 530 1.0628
276.85 550 1.0758
13.1.5 Hold the DSC test chamber isothermally at the initial
286.85 560 1.0819
temperature for at least 4 min to establish equilibrium. Record
296.85 570 1.0877
this thermal curve (refer to 12.4).
306.85 580 1.0934
316.85 590 1.0988
13.1.6 Heat the test specimen from the initial to final
326.85 600 1.1040
temperature at a rate of 20 °C/min. Continue to record the
336.85 610 1.1090
thermal curve.
346.85 620 1.1138
356.85 630 1.1184
NOTE 8—The precision of this test method is enhanced by this high
366.85 640 1.1228
heating rate. Other heating rates may be used but shall be reported.
376.85 650 1.1272
386.85 660 1.1313
13.1.7 Record a steady-state isothermal baseline at the
396.85 670 1.1353
upper temperature limit. Refer to 12.4.
406.85 680 1.1393
416.85 690 1.1431
13.1.7.1 Terminate the thermal curve after this period.
426.85 700 1.1467
13.1.7.2 Cool the DSC test chamber to ambient tempera-
446.85 720 1.1538
ture.
466.85 740 1.1605
486.85 760 1.1667
13.1.8 Place the sapphire standard into the same specimen
506.85 780 1.1727
holder plus lid used in 13.1.2.
526.85 800 1.1784
13.1.9 Weigh sapphire standard and specimen holder plus
546.85 820 1.1839
566.85 840 1.1890
lid to a precision of 60.01 mg and record the weight.
586.85 860 1.1939
13.1.10 Follow 13.1.4-13.1.7.
606.85 880 1.1986
626.85 900 1.2031
NOTE 9—In the manual method, scan the sapphire standard at the same
636.85 910 1.2053
sensitivity as used for the empty specimen holder.
646.85 920 1.2074
NOTE 10—The procedure (13.1.1-13.1.9) may be performed at some
666.85 940 1.2116
regular interval, or prior to every specific heat capacity determination of
686.85 960 1.2155
706.85 980 1.2194
test specimens. Refer to 11.5.
726.85 1000 1.2230
13.2 Unknown Specimens—13.1.1-13.1.7.
A 3
See Ref (1).
NOTE 11—Calculations are simplified if the same specimen holder is
used for the empty specimen holder and the specimen plus specimen
E 1269
holder
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.