ASTM E2781-11
(Practice)Standard Practice for Evaluation of Methods for Determination of Kinetic Parameters by Thermal Analysis
Standard Practice for Evaluation of Methods for Determination of Kinetic Parameters by Thermal Analysis
SIGNIFICANCE AND USE
The kinetic parameters provided in this standard may be used to evaluate the performance of a standard, apparatus, techniques or software for the determination parameters (such as Test Methods E698, E1641, E2041, or E2070) using thermal analysis techniques such as differential scanning calorimetry, and accelerating rate calorimetry (Guide E1981). The results obtained by these approaches may be compared to the values provided by this practice.
Note 4—Not all reference materials are suitable for each measurement technique.
SCOPE
1.1 It is the purpose of this Practice to provide kinetic parameters for reference materials used for evaluation of thermal analysis methods, apparatus and software where enthalpy and temperature are measured. This Practice addresses both exothermic and endothermic, nth order and autocatalytic reactions.
1.2 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.3 There is no International Organization for Standardization (ISO) equivalent to this standard.
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: E2781 − 11
StandardPractice for
Evaluation of Methods for Determination of Kinetic
Parameters by Thermal Analysis
This standard is issued under the fixed designation E2781; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope and Daniels Method
E2070Test Method for Kinetic Parameters by Differential
1.1 It is the purpose of this practice to provide kinetic
Scanning Calorimetry Using Isothermal Methods
parameters for reference materials used for evaluation of
thermal analysis methods, apparatus and software where en-
3. Terminology
thalpy and temperature are measured. This practice addresses
3.1 Definitions—Specific technical terms used in this prac-
both exothermic and endothermic, nth order and autocatalytic
tice are defined in Terminologies E473 and E1142, including
reactions.
differential scanning calorimetry.
1.2 The values stated in SI units are to be regarded as
4. Summary of Practice
standard. No other units of measurement are included in this
standard.
4.1 Kinetics is the study of the relationship of the extent of
a chemical reaction to the independent parameters of time and
1.3 There is no International Organization for Standardiza-
temperature. This relationship is often described using the
tion (ISO) equivalent to this standard.
Arrhenius expression where:
1.4 This standard does not purport to address all of the
dα/dt 5Zf α exp 2E/RT (1)
safety concerns, if any, associated with its use. It is the ~ ! ~ !
responsibility of the user of this standard to establish appro-
where:
priate safety and health practices and determine the applica-
α = fraction left to react,
bility of regulatory limitations prior to use.
f(α) = some function of (α),
E = activation energy (J/mol),
2. Referenced Documents
–1 –1
R = gas constant (=8.314J mol K ),
2.1 ASTM Standards:
T = absolute temperature (K), and
E473Terminology Relating to Thermal Analysis and Rhe- Z = pre-exponential factor (1/sec).
ology
4.2 For many reactions of interest the description of the
E698Test Method for Arrhenius Kinetic Constants for
function of amount left to react is of the form:
Thermally Unstable Materials Using Differential Scan-
m n
f~α! 5α ~1 2α! (2)
ning Calorimetry and the Flynn/Wall/Ozawa Method
E1142Terminology Relating to Thermophysical Properties
where m and n are the overall reaction orders. This form of
E1641TestMethodforDecompositionKineticsbyThermo-
the concentration dependence is known as the auto-catalytic
gravimetry Using the Ozawa/Flynn/Wall Method
form or the Sestak-Berggren reaction (1). If the value of m
n
E1981Guide for Assessing Thermal Stability of Materials
equals 0, then f(α) reduces to the form of f(α)=(1–α)
by Methods of Accelerating Rate Calorimetry
commonly call nth order reaction.
E2041Test Method for Estimating Kinetic Parameters by
4.3 Eq 1 may be evaluated in either its exponential or
Differential Scanning Calorimeter Using the Borchardt
logarithmic form:
ln dα/dt 5 lnZ1ln f α 2 E/RT (3)
~ ! ~ ~ !!
This practice is under the jurisdiction of ASTM Committee E37 on Thermal
4.4 The study of kinetics involves the determination of
Measurements and is the direct responsibility of Subcommittee E37.02 on Standard
values of E, Z, m, and n for a given reaction.
Reference Materials.
Current edition approved March 1, 2011. Published April 2011. DOI: 10.1520/
NOTE1—Activationenergyandpre-exponentialfactorarenotindepen-
E2781-11.
dent parameters but are inter-related.
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Standards volume information, refer to the standard’s Document Summary page on The boldface numbers in parentheses refer to a list of references at the end of
the ASTM website. this standard.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
E2781 − 11
NOTE 2—The descriptions provided in Eq 1-3 are only mathematical NOTE 4—Not all reference materials are suitable for each measurement
models. That is, they represent the fitting of mathematical equations to technique.
often “noisy” experimental data. In practice no such model will faithfully
describe the complete reaction(s) under all conditions for the materials
6. Hazards
described in this practice.
6.1 Thermally reactive materials evolve heat as part of the
4.5 Values for the kinetic parameter are typically in the
indicated reaction. Build up of this heat may lead to a
ranges indicated below:
dangerous over-pressure condition or to a self accelerating
–1
log Z: 8 to 30 with Z in s
reaction. Operators shall use caution when working with such
E: 50 to 250 kJ/mol
materials.Operatorsshalluseassmallamountofmaterialasis
n: 0.0 to 2.0
m: 0 to 2.0
practical for the measurement.
4.6 By their nature, thermally reactive materials may
6.2 The reference materials described in this standard and
change with time. For this reason, certified reference materials
their decomposition products may be explosive, carcinogenic,
arenotavailableforuseintheevaluationofkineticparameters.
hazardous, toxic, or corrosive. Handling of these materials
The user of this standard may synthesize or purchase from a
should be performed by trained workers who are knowledge-
commercial laboratory supply house materials of suitable
able with the Material Safety Data Sheets (MSDS) for each
purity for use in this standard.
material. Tetramethyl succinonitrile (TMSN), a decomposition
product of azobisisobutyronitrile (AIBN), is considered a very
NOTE 3—Storage of reference materials in a refrigerator may prolong
toxic (neurotoxic agent) and hazardous substance.
shelf life. Observe manufacturers recommendations.
4.7 The recommended values for the thermal active mate-
7. Procedure
rials identified in this standard are taken from “best values”
7.1 Experimentally determined kinetic parameters are com-
found in the open literature as described in the accompanying
tables. pared to the values described in this practice as their quotient,
expressed as percent. Thus values less than unity or 100%
5. Significance and Use indicate that the determined value is less than the reference
valuewhilethosegreaterthanunityor100 %indicatethatthe
5.1 Thekineticparametersprovidedinthisstandardmaybe
determined value is greater than the reference value.
used to evaluate the performance of a standard, apparatus,
techniques or software for the determination parameters (such
8. Calculation
asTestMethodsE698,E1641,E2041,orE2070)usingthermal
(ObservedValue×100%)
8.1 Conformance= ⁄(ReferencedValue)
analysis techniques such as differential scanning calorimetry,
and accelerating rate calorimetry (Guide E1981). The results
NOTE 5—Generally speaking, experimentally determined kinetic pa-
obtained by these approaches may be compared to the values
rameters E and log Z are considered to be in agreement if they have
conformance between 80 and 120% of the values described in Table 1.
provided by this practice.
TABLE 1 Kinetic Parameters for Kinetic Reference Materials (Derived from Tables 2-6)
NOTE 1—where:
E = activation energy,
Z = pre-exponential factor,
n = reaction order,
m = reaction order,
H = enthalpy of reaction of the pure material, and
DSC = differential scanning calorimeters.
Material E, kJ/mol log (Z,1/s) nm H, kJ/g Description
Di-t-butylperoxide Generally tested in
liquidformasa10to
20 %
solution in toluene.
158.1 15.80 1.0 0.0 1.34
Kinetic parameters are
solvent
sensitive. Suitable for
calorimeters.
Azidotriphenylmethane 165.1 19.00 1.0 0.0 Suitable for DSC.
Azobenzene Solid material, endo-
102.5 11.98 1.0 0.0 0.254 thermic; Suitable for
DSC.
Azobisisobutyronitrile Suitable for calorim-
128.5 15.12 1.0 0.0
eters and DSC.
Phenyltetrazolthiol 143 20.4 1.7 1.3 Suitable for DSC.
E2781 − 11
Experimentally determined values m and n are considered to be in
10. Precision and Bias
agreement if they have conformance between 70 and 130% of the values
described in Table 1. 10.1 This practice is used to determine the bias of kinetic
NOTE 6—The value of log Z depends upon the concentration of the
values determined by other standards or candidate standards.
reactant.
10.2 This practice does not generate experimental data and
9. Report
has no precision.
9.1 Identification of the kinetic method being examined.
9.1.1 Identification of the reference material being used for
11. Keywords
the comparison, its source and purity.
11.1 activation energy; kinetics; pre-exponential factor; re-
9.1.2 The comparison quotient (conformance) for each ki-
action order; thermal analysis
netic parameter.
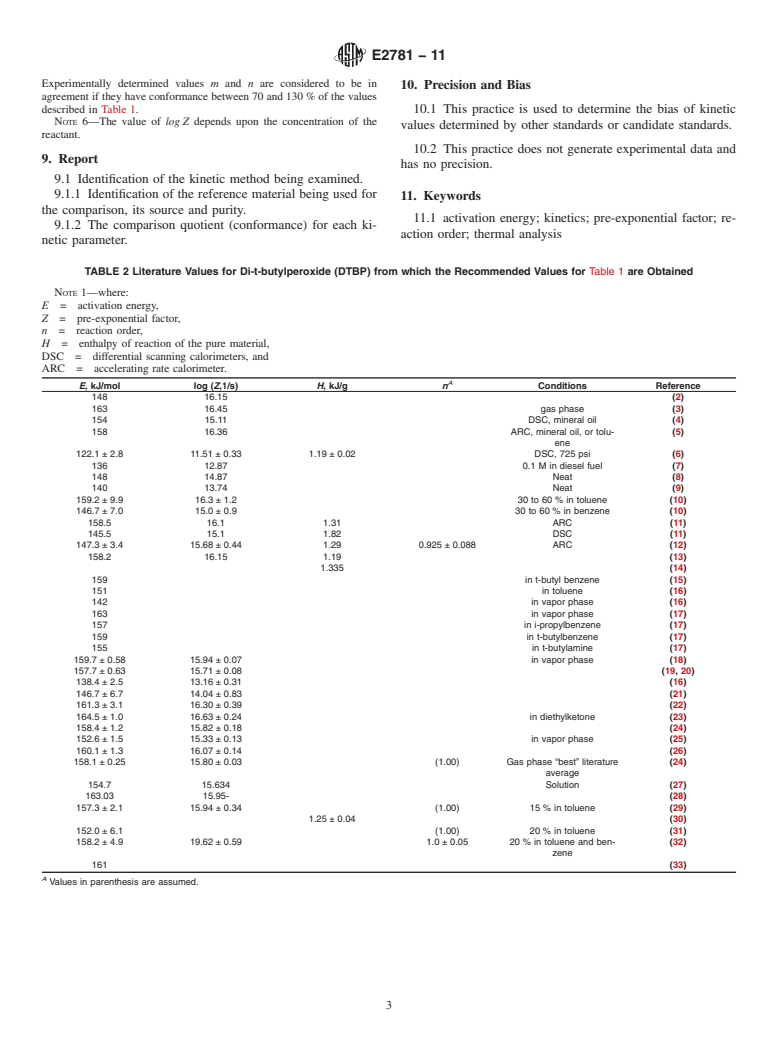
TABLE 2 Literature Values for Di-t-butylperoxide (DTBP) from which the Recommended Values for Table 1 are Obtained
NOTE 1—where:
E = activation energy,
Z = pre-exponential factor,
n = reaction order,
H = enthalpy of reaction of the pure material,
DSC = differential scanning calorimeters, and
ARC = accelerating rate calorimeter.
A
E, kJ/mol log (Z,1/s) H, kJ/g n Conditions Reference
148 16.15 (2)
163 16.45 gas phase (3)
154 15.11 DSC, mineral oil (4)
158 16.36 ARC, mineral oil, or tolu- (5)
ene
122.1 ± 2.8 11.51 ± 0.33 1.19 ± 0.02 DSC, 725 psi (6)
136 12.87 0.1 M in diesel fuel (7)
148 14.87 Neat (8)
140 13.74 Neat (9)
159.2 ± 9.9 16.3 ± 1.2 30 to 60 % in toluene (10)
146.7 ± 7.0 15.0 ± 0.9 30 to 60 % in benzene (10)
158.5 16.1 1.31 ARC (11)
145.5 15.1 1.82 DSC (11)
147.3 ± 3.4 15.68 ± 0.44 1.29 0.925 ± 0.088 ARC (12)
158.2 16.15 1.19 (13)
1.335 (14)
159 in t-butyl benzene (15)
151 in toluene (16)
142 in vapor phase (16)
163 in vapor phase (17)
157 in i-propylbenzene (17)
159 in t-butylbenzene (17)
155 in t-butylamine (17)
159.7 ± 0.58 15.94 ± 0.07 in vapor phase (18)
157.7 ± 0.63 15.71 ± 0.08 (19, 20)
138.4 ± 2.5 13.16 ± 0.31 (16)
146.7 ± 6.7 14.04 ± 0.83 (21)
161.3 ± 3.1 16.
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.