ASTM F2182-02
(Test Method)Standard Test Method for Measurement of Radio Frequency Induced Heating Near Passive Implants During Magnetic Resonance Imaging
Standard Test Method for Measurement of Radio Frequency Induced Heating Near Passive Implants During Magnetic Resonance Imaging
SCOPE
1.1 This test method covers measurement of Radio Frequency (RF) induced heating near a passive medical implant and its surroundings during Magnetic Resonance Imaging (MRI).
1.2 This test method is one of those required to determine if the presence of a passive implant may cause injury to the person with the implant during an MRI procedure. Other safety issues that should be addressed include magnetically induced displacement force and torque.
1.3 The amount of RF-induced temperature rise for a given specific absorption rate (SAR) will depend on the RF frequency, which is proportional to the static magnetic field strength. Because of possible additional heating, particularly when device dimensions exceed a quarter wavelength, conclusions from measurements made at one frequency may not apply to other frequencies.
1.4 This test method assumes that testing is done on devices that will be entirely inside the body.
1.5 This test method applies to whole body magnetic resonance equipment, as defined in section 2.2.103 of the IEC Standard 60601-2-33 with a whole body RF transmit coil as defined in section 2.2.100. The RF coil is assumed to have quadrature excitation.
1.6 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
Designation: F 2182 – 02
Standard Test Method for
Measurement of Radio Frequency Induced Heating Near
Passive Implants During Magnetic Resonance Imaging
This standard is issued under the fixed designation F 2182; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope Magnetic Resonance Environment
F 2119 Test Method for Evaluation of MR Image Artifacts
1.1 This test method covers measurement of Radio Fre-
from Passive Implants
quency (RF) induced heating near a passive medical implant
2.2 IEC Standard:
and its surroundings during Magnetic Resonance Imaging
60601-2-33 Medical Electrical Equipment—Part 2: Particu-
(MRI).
lar Requirements for the Safety of Magnetic Resonance
1.2 This test method is one of those required to determine if
Equipment for Medical Diagnosis, 2001
the presence of a passive implant may cause injury to the
person with the implant during an MRI procedure. Other safety
3. Terminology
issues that should be addressed include magnetically induced
3.1 Definitions—For the purposes of this test method, the
displacement force and torque.
definitions in 3.1.1-3.1.8 shall apply.
1.3 The amount of RF-induced temperature rise for a given
3.1.1 isocenter—geometric center of the gradient coil sys-
specific absorption rate (SAR) will depend on the RF fre-
tem, which generally is the geometric center of a scanner with
quency, which is proportional to the static magnetic field
a cylindrical bore.
strength. Because of possible additional heating, particularly
3.1.2 magnetic resonance imaging (MRI)—diagnostic im-
when device dimensions exceed a quarter wavelength, conclu-
aging technique that uses static and time varying magnetic
sions from measurements made at one frequency may not
fields to provide images of tissue by the magnetic resonance of
apply to other frequencies.
nuclei.
1.4 This test method assumes that testing is done on devices
3.1.3 medical implant—a structure or device that is placed
that will be entirely inside the body.
within the body of the patient for medical diagnostic or
1.5 This test method applies to whole body magnetic
therapeutic purposes.
resonance equipment, as defined in section 2.2.103 of the IEC
3.1.4 MR safe—the device, when used in the MR environ-
Standard 60601-2-33 with a whole body RF transmit coil as
ment, has been demonstrated to present no additional risk to
defined in section 2.2.100. The RF coil is assumed to have
the patient or other individuals, but may affect the quality of
quadrature excitation.
the diagnostic information. The MR conditions in which the
1.6 This standard does not purport to address all of the
device was tested should be specified in conjunction with the
safety concerns, if any, associated with its use. It is the
terms MR safe and MR compatible since a device which is safe
responsibility of the user of this standard to establish appro-
or compatible under one set of conditions may not be found to
priate safety and health practices and determine the applica-
be so under more extreme MR conditions.
bility of regulatory limitations prior to use.
3.1.5 MR compatible—the device, when used in the MR
2. Referenced Documents environment, is MR safe and has been demonstrated to neither
significantly affect the quality of the diagnostic information nor
2.1 ASTM Standards:
have its operations affected by the MR device. The MR
A 340 Terminology of Symbols and Definitions Relating to
conditions in which the device was tested should be specified
Magnetic Testing
in conjunction with the terms MR safe and MR compatible
F 2052 Test Method for Measurement of Magnetically In-
since a device which is safe or compatible under one set of
duced Displacement Force on Passive Implants in the
conditions may not be found to be so under more extreme MR
conditions.
3.1.6 passive implant—an implant that serves its function
This test method is under the jurisdiction of ASTM Committee F04 on Medical
without supply of electrical power.
and Surgical Materials and Devices and is the direct responsibility of Subcommittee
F04.15 on Material Test Methods.
Current edition approved Apr. 10, 2002. Published June 2002. Annual Book of ASTM Standards, Vol 13.01.
2 4
Annual Book of ASTM Standards, Vol 03.04. Available from the International Electrotechnical Commission (IEC), 3 rue de
Varembe, Case postale 131, CH-1211 Geneva 20, Switzerland.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
NOTICE: This standard has either been superseded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
F 2182
NOTE 1—The device does not have to be sterile at the time of testing.
3.1.7 radio frequency (RF) magnetic field—the magnetic
However, it should have been subjected to all processing, packaging, and
field in MRI that is used to flip the magnetic moments. The
sterilization steps before testing because any of these steps may affect the
frequency of the RF field is gB where g is the gyromagnetic
magnetic properties of the device.
constant, 42.56 MHz/T for protons, and B is the static
7.2 For purposes of device qualification, implant devices
magnetic field in Tesla.
shall not be altered in any manner prior to testing.
3.1.8 specific absorption rate (SAR)—the mass normalized
7.3 This test method may be used on prototype devices
rate at which RF energy is deposited in biological tissue. SAR
during product development.
is typically indicated in W/kg.
8. Procedure
4. Summary of Test Method
8.1 Phantom Morphology—Use a phantom geometry that
4.1 The implant to be tested is placed in a phantom material
reflects how the implant is placed in the body. The phantom
that simulates the electrical and thermal properties of the
container needs to be large enough to allow the device to be
human body. The phantom material will include saline solution
placed in a position representative of where it would be in the
and a gelling agent. Fiber optic temperature probes are placed
body. The container and all its parts should be made of material
at locations where the induced heating is expected to be
that is an electrical insulator and is non-magnetic. A whole
greatest. The phantom is placed in an MR system with a
body phantom should simulate the RF loading that would occur
cylindrical bore or an apparatus that reproduces the RF field of
with a patient. The phantom should have the general shape of
this type of system. An RF field with SAR of at least 1 W/kg
a patient (Fig. 1) but a rectangular phantom (Fig. 2) is also
averaged over the volume of the phantom is applied. The
acceptable. For application of RF by the body coil, the
temperature rise at the sensors is measured during the approxi-
phantom should contain at least 30 kg of phantom material. For
mately 15 min of RF application, or other appropriate period,
an implant inserted entirely in the head, a spherical phantom
depending on the mass and thermal conductivity of critical
with dimensions similar to those of the human head may be
parts of the device. Temperature measurements at one or more
appropriate. Generally, a homogeneous phantom will suffice,
locations away from the device serve as the control.
but in certain cases it may be appropriate to incorporate
materials of different conductivity within the phantom.
5. Significance and Use
8.2 Phantom Material—Phantom materials simulating tis-
5.1 This test method describes a test procedure for evaluat-
sue for the RF heating test during MRI shall meet the following
ing the RF-induced temperature rise in MRI in the vicinity of
criteria.
an implanted medical device. The actual temperature rise in the
8.2.1 Conductivity—Conductivity shall be 0.4 to 0.8 S/m at
patient will depend on a variety of factors beyond the SAR and
64 MHz, depending on the tissue to be modeled. (See Stuchly
time of RF application. The conditions and results of the
et al. (1) for data on tissue electrical properties and Athey et
testing should be included in the device labeling so that the
al. (2) for procedures for measurement of electrical properties.)
attending physician can make the decision of whether to allow
Electrical conductivity at low frequency will be less than at 64
the patient with the implant to undergo an MRI procedure.
MHz. The phantom conductivity should be 0.2 to 0.4 S/m for
6. Apparatus measurements made at a frequency of 1 kHz. (Stuchly and
Stuchly (3)).
6.1 Test Apparatus—The test apparatus consists of a suit-
8.2.2 Dielectric Constant—Dielectric constant shall be 60
able phantom and an MR imager for production of the RF field.
to 100 at 64 MHz.
The phantom, implant and MR imager are to simulate the
8.2.3 Thermal Parameters—The phantom material shall
electrical and physical environment that the patient and device
have thermal properties similar to those of the body which has
experience during an MRI procedure.
-7 2
diffusivity of about 1.3 3 10 m /s and heat capacity close to
6.2 Temperature Sensor—A suitable temperature measuring
that of water, 4184 J/kg °C.
device, usually a fiber optic probe, is used to measure tempera-
8.2.4 Viscosity—The viscosity shall be sufficient so that the
ture versus time of RF exposure in the vicinity of the implant.
phantom material does not allow bulk transport or convection
The temperature sensor will have a resolution of 0.1°C and a
currents. Generally, this is achieved by inclusion of a gelling
sensitive volume not to exceed 1 mm in radius. Fluoroptic
agent.
temperature probes have been found to be satisfactory for this
8.3 Phantom Formulation—A suitable gelled phantom
purpose.
(Rezai (4)) can be made with 0.8 g/L NaCl and 5.85 g/L
7. Test Specimens
Polyacrylic acid into distilled water. This formulation has a
room temperature conductivity of about 0.25 S/m and a
7.1 For purposes of device qualification, the implant or
viscosity sufficient to prevent convective heat transport. A
device evaluated according to this test method shall be repre-
number of other phantom formulations may be appropriate and
sentative of a finished sterilized device. For the purposes of
some are described in the rationale.
device qualification, the device evaluated according to this test
method should be a finished sterilized device.
The phantom in Fig. 2 may be purchased from Fab Lab Inc., Suite 1501325
Armstrong Rd., Northfield, MN 55057, tel. +1-507-645-2815, cbenson@fablab.net.
5 7
Particularly suitable are the Luxtron (Luxtron Corporation, Santa Clara, CA, The boldface numbers in parentheses refer to the list of references at the end of
USA) Models 790, 3000, and 3100 Fluoroptic Thermometer Systems and the 0.6 this standard.
mm diameter SFF-10 probe. Catalog 43,636-4, Aldrich Chemical Company, Inc., Milwaukee, WI, USA.
NOTICE: This standard has either been superseded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
F 2182
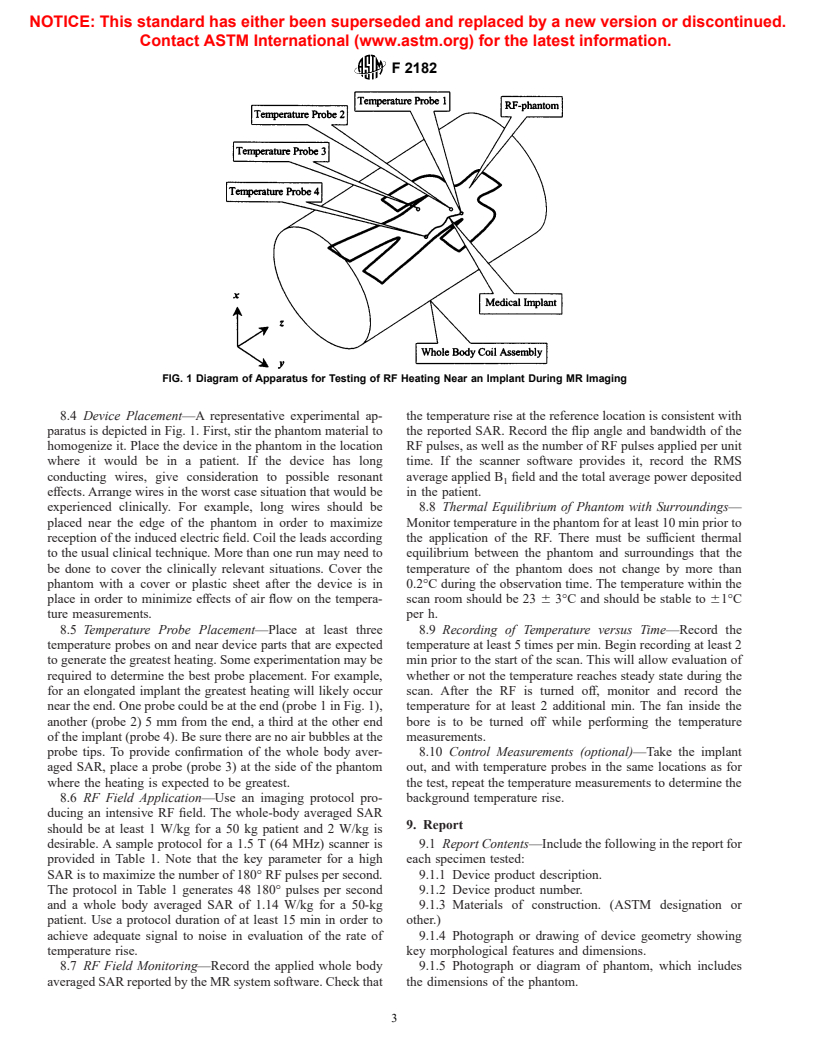
FIG. 1 Diagram of Apparatus for Testing of RF Heating Near an Implant During MR Imaging
8.4 Device Placement—A representative experimental ap- the temperature rise at the reference location is consistent with
paratus is depicted in Fig. 1. First, stir the phantom material to the reported SAR. Record the flip angle and bandwidth of the
homogenize it. Place the device in the phantom in the location RF pulses, as well as the number of RF pulses applied per unit
where it would be in a patient. If the device has long time. If the scanner software provides it, record the RMS
conducting wires, give consideration to possible resonant average applied B field and the total average power deposited
effects. Arrange wires in the worst case situation that would be in the patient.
experienced clinically. For example, long wires should be 8.8 Thermal Equilibrium of Phantom with Surroundings—
placed near the edge of the phantom in order to maximize Monitor temperature in the phantom for at least 10 min prior to
reception of the induced electric field. Coil the leads according the application of the RF. There must be sufficient thermal
to the usual clinical technique. More than one run may need to equilibrium between the phantom and surroundings that the
be done to cover the clinically relevant situations. Cover the temperature of the phantom does not change by more than
phantom with a cover or plastic sheet after the device is in 0.2°C during the observation time. The temperature within the
place in order to minimize effects of air flow on the tempera- scan room should be 23 6 3°C and should be stable to 61°C
ture measurements. per h.
8.5 Temperature Probe Placement—Place at least three 8.9 Recording of Temperature versus Time—Record the
temperature probes on and near device parts that are expected temperature at least 5 times per min. Begin recording at least 2
to generate the greatest heating. Some experimentation may be min prior to the start of the scan. This will allow evaluation of
required to determine the best probe placement. For example, whether or not the temperature reaches steady state during the
for an elongated implant the greatest heating will likely occur scan. After the RF is turned off, monitor and record the
near the end. One probe could be at the end (probe 1 in Fig. 1), temperature for at least 2 additional min. The fan inside the
another (probe 2) 5 mm from the end, a third at the other end bore is to be turned off while performing the temperature
of the implant (probe 4). Be sure there are no air bubbles at the measurements.
probe tips. To provide confirmation of the whole body aver- 8.10 Control Measurements (optional)—Take the implant
aged SAR, place a probe (probe 3) at the side of the phantom out, and with temperature probes in the same locations as for
where the heating is expected to be greatest. the test, repeat the temperature measurements to determine the
8.6 RF Field Application—Use an imaging protocol pro- background temperature rise.
ducing an intensive RF field. The whole-body averaged SAR
9. Report
should be at least 1 W/kg for a 50 kg patient and 2 W/kg is
desirable. A sample protocol for a 1.5 T (64 MHz) scanner is 9.1 Report Contents—Include the following in the report for
provided in Table 1. Note that the key parameter for a high each specimen tested:
SAR is to maximize the number of 180° RF pulses per second. 9.1.1 Device product description.
The protocol in Table 1 generates 48 180° pulses per second 9.1.2 Device product number.
and a whole body
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.