ASTM F2977-20
(Test Method)Standard Test Method for Small Punch Testing of Polymeric Biomaterials Used in Surgical Implants
Standard Test Method for Small Punch Testing of Polymeric Biomaterials Used in Surgical Implants
SIGNIFICANCE AND USE
4.1 Miniature specimen testing techniques are used to characterize the mechanical behavior of polymer stock materials and surgical implants after manufacture, sterilization, shelf aging, radiation crosslinking, thermal treatment, filler incorporation, and implantation (1-3). Furthermore, experimental materials can be evaluated after accelerated aging, fatigue testing, and hip, knee, or spine wear simulation. Consequently, the small punch test makes it possible to examine relationships between wear performance and mechanical behavior. This test method can also be used to rank the mechanical behavior relative to a reference control material.
4.2 Small punch testing results may vary with specimen preparation and with the speed and environment of testing. Consequently, where precise comparative results are desired, these factors must be carefully controlled.
SCOPE
1.1 This test method covers the determination of mechanical behavior of polymeric biomaterials by small punch testing of miniature disk specimens (0.5 mm in thickness and 6.4 mm in diameter). The test method has been established for characterizing surgical materials after ram extrusion or compression molding (1-3)2; for evaluating as-manufactured implants and sterilization method effects (4, 5); as well as for testing of implants that have been retrieved (explanted) from the human body (6, 7).
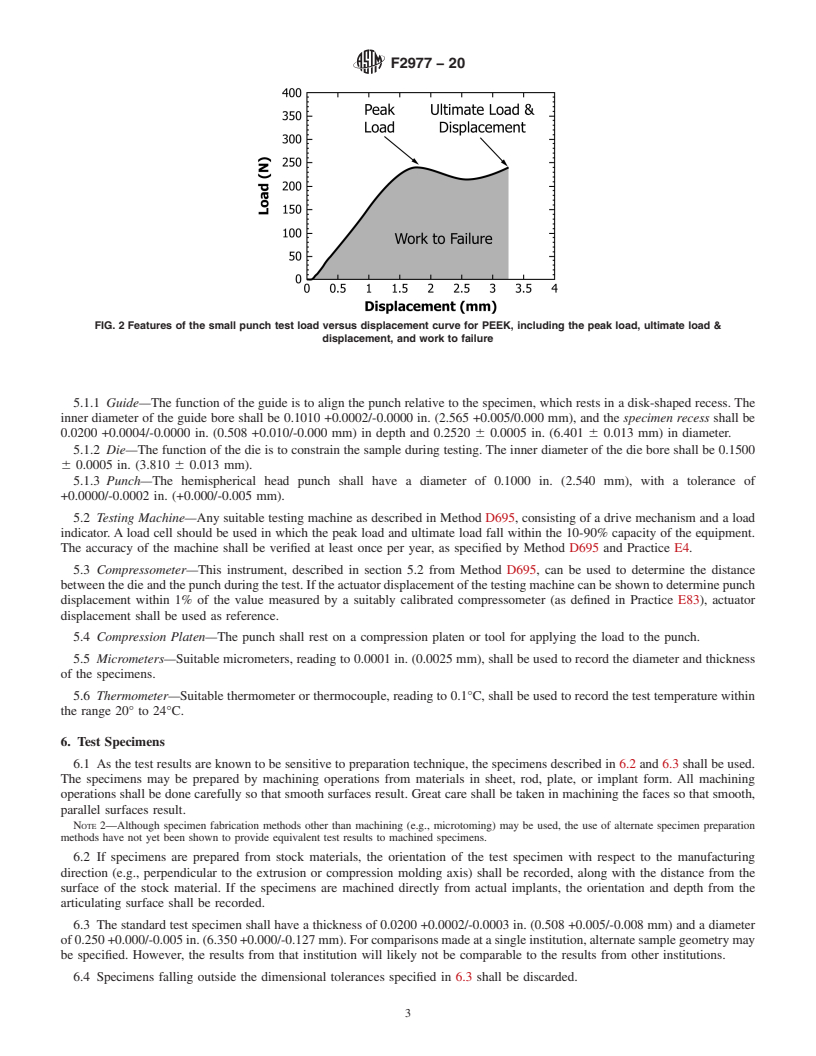
1.2 The results of the small punch test, namely the peak load, ultimate displacement, ultimate load, and work to failure, provide metrics of the yielding, ultimate strength, ductility, and toughness under multiaxial loading conditions. Because the mechanical behavior can be different when loaded under uniaxial and multiaxial loading conditions (8), the small punch test provides a complementary mechanical testing technique to the uniaxial tensile test. However, it should be noted that the small punch test results may not correlate with uniaxial tensile test results.
1.3 In addition to its use as a research tool in implant retrieval analysis, the small punch test can be used as a laboratory screening test to evaluate new materials with minimal material waste (1).
1.4 The small punch test has been applied to other polymers, including polymethyl methacrylate (PMMA) bone cement, polyacetal, and high density polyethylene (HDPE), ultra high molecular weight polyethylene (UHMWPE), and polyetheretherketone (PEEK) (2, 3, 5, 9, 10). This standard outlines general guidelines for the small punch testing of implantable polymers.
1.5 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of regulatory limitations prior to use.
1.6 This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
General Information
Buy Standard
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
Designation: F2977 − 20
Standard Test Method for
Small Punch Testing of Polymeric Biomaterials Used in
1
Surgical Implants
This standard is issued under the fixed designation F2977; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope responsibility of the user of this standard to establish appro-
priate safety, health, and environmental practices and deter-
1.1 Thistestmethodcoversthedeterminationofmechanical
mine the applicability of regulatory limitations prior to use.
behavior of polymeric biomaterials by small punch testing of
1.6 This international standard was developed in accor-
miniature disk specimens (0.5 mm in thickness and 6.4 mm in
dance with internationally recognized principles on standard-
diameter). The test method has been established for character-
ization established in the Decision on Principles for the
izing surgical materials after ram extrusion or compression
2 Development of International Standards, Guides and Recom-
molding (1-3) ; for evaluating as-manufactured implants and
mendations issued by the World Trade Organization Technical
sterilization method effects (4, 5); as well as for testing of
Barriers to Trade (TBT) Committee.
implants that have been retrieved (explanted) from the human
body (6, 7).
2. Referenced Documents
1.2 The results of the small punch test, namely the peak
3
2.1 ASTM Standards:
load, ultimate displacement, ultimate load, and work to failure,
D695 Test Method for Compressive Properties of Rigid
providemetricsoftheyielding,ultimatestrength,ductility,and
Plastics
toughness under multiaxial loading conditions. Because the
D883 Terminology Relating to Plastics
mechanical behavior can be different when loaded under
E4 Practices for Force Verification of Testing Machines
uniaxial and multiaxial loading conditions (8), the small punch
E83 Practice for Verification and Classification of Exten-
test provides a complementary mechanical testing technique to
someter Systems
the uniaxial tensile test. However, it should be noted that the
E177 Practice for Use of the Terms Precision and Bias in
small punch test results may not correlate with uniaxial tensile
ASTM Test Methods
test results.
E691 Practice for Conducting an Interlaboratory Study to
1.3 In addition to its use as a research tool in implant
Determine the Precision of a Test Method
retrieval analysis, the small punch test can be used as a
F1714 Guide for GravimetricWearAssessment of Prosthetic
laboratory screening test to evaluate new materials with
Hip Designs in Simulator Devices
minimal material waste (1).
F1715 Guide for Wear Assessment of Prosthetic Knee De-
4
1.4 Thesmallpunchtesthasbeenappliedtootherpolymers, signs in Simulator Devices (Withdrawn 2006)
F2003 Practice for Accelerated Aging of Ultra-High Mo-
including polymethyl methacrylate (PMMA) bone cement,
polyacetal, and high density polyethylene (HDPE), ultra high lecular Weight Polyethylene after Gamma Irradiation in
Air
molecular weight polyethylene (UHMWPE), and polyethere-
therketone (PEEK) (2, 3, 5, 9, 10). This standard outlines F2102 Guide for Evaluating the Extent of Oxidation in
Polyethylene Fabricated Forms Intended for Surgical
general guidelines for the small punch testing of implantable
polymers. Implants
1.5 This standard does not purport to address all of the
3. Terminology
safety concerns, if any, associated with its use. It is the
3.1 Definitions:
1
This test method is under the jurisdiction ofASTM Committee F04 on Medical
and Surgical Materials and Devices and is the direct responsibility of Subcommittee
3
F04.15 on Material Test Methods. For referenced ASTM standards, visit the ASTM website, www.astm.org, or
Current edition approved April 1, 2020. Published June 2020. Originally contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
ɛ1
approved in 2013. Last previous edition approved in 2013 as F2977–13 . DOI: Standards volume information, refer to the standard’s Document Summary page on
10.1520/F2977-20. the ASTM website.
2 4
The boldface numbers in parentheses refer to the list of references at the end of The last approved version of this historical standard is referenced on
this standard. www.astm.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
F2977 − 20
3.1.1 small punch test, n—a test wherein the specimen is of
...
This document is not an ASTM standard and is intended only to provide the user of an ASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
´1
Designation: F2977 − 13 F2977 − 20
Standard Test Method for
Small Punch Testing of Polymeric Biomaterials Used in
1
Surgical Implants
This standard is issued under the fixed designation F2977; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1
ε NOTE—Editorial corrections were made to 5.1 in February 2020.
1. Scope
1.1 This test method covers the determination of mechanical behavior of polymeric biomaterials by small punch testing of
miniature disk specimens (0.5 mm in thickness and 6.4 mm in diameter). The test method has been established for characterizing
2
surgical materials after ram extrusion or compression molding (1-3) ; for evaluating as-manufactured implants and sterilization
method effects (4, 5); as well as for testing of implants that have been retrieved (explanted) from the human body (6, 7).
1.2 The results of the small punch test, namely the peak load, ultimate displacement, ultimate load, and work to failure, provide
metrics of the yielding, ultimate strength, ductility, and toughness under multiaxial loading conditions. Because the mechanical
behavior can be different when loaded under uniaxial and multiaxial loading conditions (8), the small punch test provides a
complementary mechanical testing technique to the uniaxial tensile test. However, it should be noted that the small punch test
results may not correlate with uniaxial tensile test results.
1.3 In addition to its use as a research tool in implant retrieval analysis, the small punch test can be used as a laboratory
screening test to evaluate new materials with minimal material waste (1).
1.4 The small punch test has been applied to other polymers, including polymethyl methacrylate (PMMA) bone cement,
polyacetal, and high density polyethylene (HDPE), ultra high molecular weight polyethylene (UHMWPE), and polyetherether-
ketone (PEEK) (2, 3, 5, 9, 10). This standard outlines general guidelines for the small punch testing of implantable polymers.
1.5 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility
of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of
regulatory limitations prior to use.
1.6 This international standard was developed in accordance with internationally recognized principles on standardization
established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued
by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
2. Referenced Documents
3
2.1 ASTM Standards:
D695 Test Method for Compressive Properties of Rigid Plastics
D883 Terminology Relating to Plastics
E4 Practices for Force Verification of Testing Machines
E83 Practice for Verification and Classification of Extensometer Systems
E177 Practice for Use of the Terms Precision and Bias in ASTM Test Methods
E691 Practice for Conducting an Interlaboratory Study to Determine the Precision of a Test Method
F1714 Guide for Gravimetric Wear Assessment of Prosthetic Hip Designs in Simulator Devices
4
F1715 Guide for Wear Assessment of Prosthetic Knee Designs in Simulator Devices (Withdrawn 2006)
1
This test method is under the jurisdiction of ASTM Committee F04 on Medical and Surgical Materials and Devices and is the direct responsibility of Subcommittee
F04.15 on Material Test Methods.
Current edition approved June 1, 2013April 1, 2020. Published August 2013.June 2020. Originally approved in 2013. Last previous edition approved in 2013 as
ɛ1
F2977–13 . DOI: 10.1520/F2977-13E01.10.1520/F2977-20.
2
The boldface numbers in parentheses refer to the list of references at the end of this standard.
3
For referenced ASTM standards, visit the ASTM website, www.astm.org, or contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM Standards
volume information, refer to the standard’s Document Summary page on the ASTM website.
4
The last approved version of this historical standard is referenced on www.astm.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
F2977 − 20
F2003 Practice for Accelerated Aging of Ultra-High Molecular W
...









Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.