ASTM D3266-91(2000)e1
(Test Method)Standard Test Method for Automated Separation and Collection of Particulate and Acidic Gaseous Fluoride in the Atmosphere (Double Paper Tape Sampler Method)
Standard Test Method for Automated Separation and Collection of Particulate and Acidic Gaseous Fluoride in the Atmosphere (Double Paper Tape Sampler Method)
SCOPE
1.1 This test method describes the automatic separation and collection on chemically treated paper tapes of particulate and gaseous forms of acidic fluorides in the atmosphere by means of a double paper tape sampler. The sampler may be programmed to collect and store individual air samples obtained over time periods from several minutes to 3 h. A 30.5-m (100-ft) tape will allow unattended operation for the automatic collection of up to 600 samples.
1.2 The values stated in SI units are to be regarded as the standard. The values given in parentheses are for information only.
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
e1
Designation:D3266–91(Reapproved2000)
Standard Test Method for
Automated Separation and Collection of Particulate and
Acidic Gaseous Fluoride in the Atmosphere (Double Paper
1,2
Tape Sampler Method)
This standard is issued under the fixed designation D 3266; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
This standard has been approved for use by agencies of the Department of Defense.
e NOTE—Editorial corrections were made throughout in September 2000.
1. Scope D 1356 Terminology Relating to Sampling and Analysis of
Atmospheres
1.1 This test method describes the automatic separation and
D 1357 Practice for Planning the Sampling of the Ambient
collection on chemically treated paper tapes of particulate and
Atmosphere
gaseous forms of acidic fluorides in the atmosphere by means
D 3195 Practice for Rotameter Calibration
of a double paper tape sampler. The sampler may be pro-
D 3268 Test Method for Separation and Collection of Par-
grammed to collect and store individual air samples obtained
ticulateandGaseousFluoridesintheAtmosphere(Sodium
over time periods from several minutes to 3 h. A30.5-m
Bicarbonate-Coated Glass Tube and Particulate Filter
(100-ft) tape will allow unattended operation for the automatic
Method)
collection of up to 600 samples.
D 3269 Test Methods for Analysis for Fluoride Content of
1.2 The values stated in SI units are to be regarded as the
the Atmosphere and Plant Tissues (Manual Procedures)
standard. The values given in parentheses are for information
D 3270 Test Methods for Analysis for Fluoride Content of
only.
the Atmosphere and Plant Tissues (Semiautomated
1.3 This standard does not purport to address all of the
Method)
safety concerns, if any, associated with its use. It is the
D 3609 Practice for Calibration Techniques Using Perme-
responsibility of the user of this standard to establish appro-
ation Tubes
priate safety and health practices and determine the applica-
D 3614 Guide for Laboratories Engaged in Sampling and
bility of regulatory limitations prior to use.
Analysis of Atmospheres and Emissions
2. Referenced Documents
3. Terminology
2.1 ASTM Standards:
3.1 Definitions—For definitions of terms used in this test
D 1071 Test Methods for Volumetric Measurement of Gas-
3 method, refer to Terminology D 1356.
eous Fuel Samples
D 1193 Specification for Reagent Water
4. Summary of Test Method
4.1 Air is drawn through an air inlet tube (see Practice
This test method is under the jurisdiction of ASTM Committee D22 on D 1357) and is first passed through an acid-treated prefilter
Sampling and Analysis of Atmospheres and is the direct responsibility of Subcom-
paper tape to remove particulate matter which may contain
mittee D22.03 on Ambient Atmospheres and Source Emissions.
fluoride and then through an alkali-treated paper tape to
Current edition approved March 15, 1991. Published May 1991. Originally
e1
remove acidic fluoride gases.
published as D 3266 – 73T. Last previous edition D 3266 – 79 .
This test method was originally adopted editorially from the Intersociety
4.2 The exhaust air is filtered through soda lime-glass wool,
CommitteeMethod 42222-02-72T;12202-04-72T,“HealthLaboratoryScience”Vol
and the cleaned air is used to pressurize the front compartment
9, No. 4, 1972, pp. 314–318. Revisions have been made to incorporate changes
to prevent fluoride contamination of the paper tapes from the
recommended by Committee D22.
ambient air.
This revision has been adapted from “Methods of Air Sampling and Analysis,”
Intersociety Committee, Edited by James P. Lodge, Jr., 3rd ed., Lewis Publishers,
Inc., 1989, pp. 352–356.
Annual Book of ASTM Standards, Vol 05.06.
4 5
Annual Book of ASTM Standards, Vol 11.01. Annual Book of ASTM Standards, Vol 11.03.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
D3266
4.3 Automatically, at the end of the preset sampling period,
the vacuum pump is turned off, the tapes are indexed, and after
indexing the vacuum pump is turned on. Indexing results in a
“dead time” of several seconds.
4.4 The paper tapes are removed from the sampler after a
selected period of operation and taken to an analytical work
area where the individual sample spots are cut out, treated to
dissolve the fluoride, and analyzed by potentiometric or pho-
6,7,8
tometric methods.
5. Significance and Use
5.1 This test method provides a means of automatically
separating and collecting atmospheric particulate and acidic
gaseous fluoride samples.
5.2 Since the samples are collected on dry tapes, the
samples are in a form which allows elution of the fluoride
content with a small volume of eluent. Consequently, the
method allows analyses of air samples taken for a time period
as short as several minutes.
6. Interferences
6.1 Particulate metallic salts, such as those of aluminum,
iron, calcium, magnesium or rare-earth elements, may react
with and remove some or all of the acidic gaseous fluoride on
the prefilter. If interfering quantities of such particulate metal-
lic salts are present, the use of Test Methods D 3268 is
FIG. 1 Dual Tape Sampler Flow Schematic
recommended because the acidic fluoride gases are collected
prior to the filter.
7. Apparatus
6.2 Acid aerosols or gases might neutralize or acidify the
7.1 The double paper tape sampler is a modification of and
alkali-treated tape and prevent quantitative uptake of the acidic
utilizes the basic principles of the sequential paper tape
fluoride gases from the atmosphere. If this potential interfer-
sampler used for dust collection. The commercially available
ence is present the decreased alkalinity of the water extract
apparatus requires modification, as described in this test
(13.2.2.1) may provide relevant information.
method, prior to use. It consists of the following:
6.3 Aluminum or certain other metals or phosphates can
7.1.1 Heated Inlet—I , TFE-fluorocarbon, 1 m (3.3 ft) in
interfere with subsequent analyses of the tapes by photometric
length, 9.5 mm ( ⁄8in.) (outside diameter), encased in a 9.5 mm
or electrometric methods. These potential interferences are
( ⁄8 in.) (inside diameter) aluminum tube. See Fig. 1. The
discussed in Test Methods D 3269 and D 3270.
aluminum jacket is wrapped in a constant wattage heating wire
6.4 There are several limitations of the test method that
of 25 W/m (8 W/ft). The tube is connected to the instrument
could possibly occur:
with a TFE-fluorocarbon fitting.
6.4.1 Although the acid-treated medium retentive prefilter
7.1.1.1 Rainshield, R —Constructed of TFE-fluorocarbon.
s
has been shown to allow passage of hydrofluoric acid, it will
7.1.1.2 Proportional Temperature Controller—H , with
restrict passage of particulate matter only as small as about 1
thermocouple reference point located at the bottom of the
µm.Thus,smallerparticulatemattermaypassthroughthefilter
sample chamber.
and impinge on or pass through the alkali-treated second tape.
7.1.1.3 Inlet Thermostat—T .
6.4.2 The maximum sampling time recommended in the
7.1.1.4 Inlet Pressure Gage—M with shutoff valve, V .
5 1
method is 3 h. This time is limited to minimize the possible
One side of the gage is connected to a TFE-fluorocarbon run
effect of particulate matter sorbing the acidic fluoride gases or
tee placed between the intake tube and the sample block, and
reducing the sampling rate.
The sole source of supply of this apparatus known to the Committee is
Anderson Samplers, Atlanta, GA. If you are aware of alternate suppliers, please
Mandl, R. H., Weinstein, L. H., Weiskopf, G. J., and Major, J. L. “The provide this information to ASTM Headquarters, 100 Barr Harbor Drive, PO Box
SeparationandCollectionofGaseousandParticulateFluorides.”PaperCP-25A,2D C700, West Conshohocken, PA 19428–2959. Your comments will recieve careful
International Clean Air Congress, Washington, DC, 1970. consideration at a meeting of the responsible technical committee, which you may
Weinstein, L. H., and Mandl, R. H. “The Separation and Collection of Gaseous attend.
and Particulate Fluorides.” VDI Berichte Nr. Vol 164, 1971, pp. 53 to 63. Zankel, K. L., McGirr, R., Romm, M. Campbell, Miller, R. “Measurement of
Lodge, James P. Jr., ed., “Methods ofAir Sampling andAnalysis,” Intersociety Ambient Ground-Level Concentrations of Hydrogen Fluoride,” Journal of The Air
Committee, 3rd ed., Lewis Publishers, Inc., 1988, pp. 352–356. Pollution Control Association, Vol 37: 1191–1196 (1987).
D3266
the other side is connected to a TFE-fluorocarbon run tee lower block shall be lowered by means of an electric solenoid
placed at the entrance to the intake tubing. which counteracts the spring pressure.
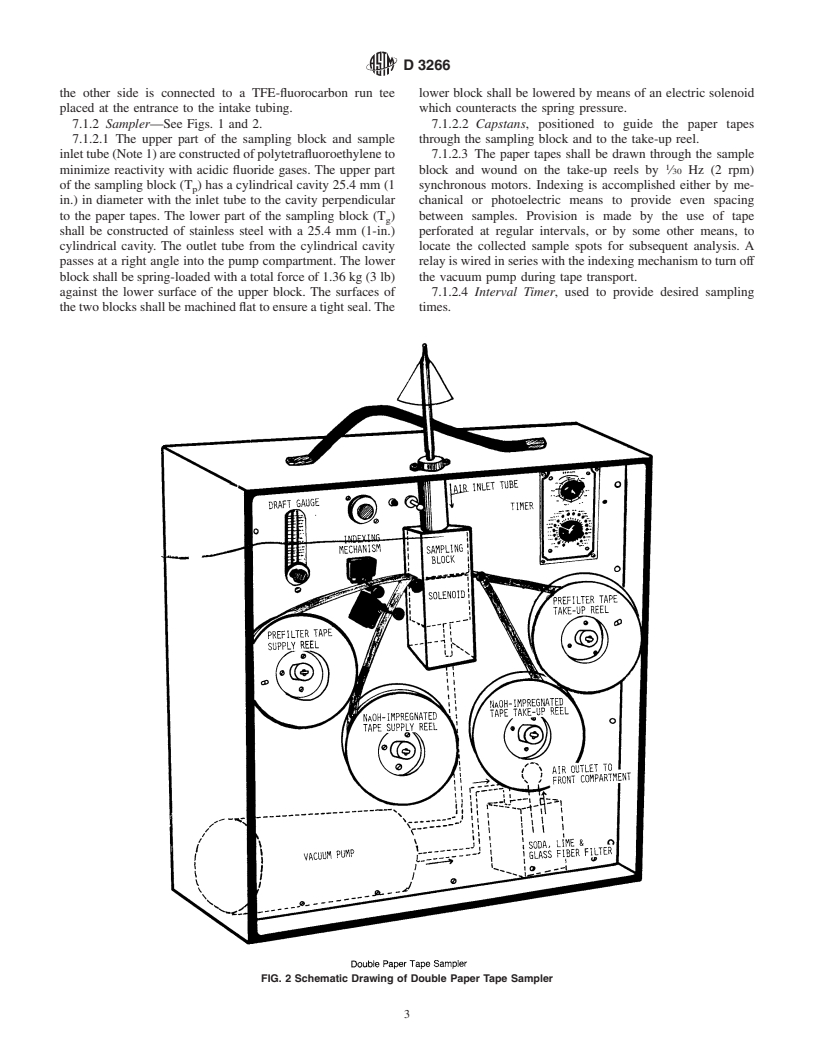
7.1.2 Sampler—See Figs. 1 and 2. 7.1.2.2 Capstans, positioned to guide the paper tapes
7.1.2.1 The upper part of the sampling block and sample through the sampling block and to the take-up reel.
inlettube(Note1)areconstructedofpolytetrafluoroethyleneto 7.1.2.3 The paper tapes shall be drawn through the sample
minimize reactivity with acidic fluoride gases. The upper part block and wound on the take-up reels by ⁄30 Hz (2 rpm)
of the sampling block (T ) has a cylindrical cavity 25.4 mm (1 synchronous motors. Indexing is accomplished either by me-
p
in.) in diameter with the inlet tube to the cavity perpendicular chanical or photoelectric means to provide even spacing
to the paper tapes. The lower part of the sampling block (T ) between samples. Provision is made by the use of tape
g
shall be constructed of stainless steel with a 25.4 mm (1-in.) perforated at regular intervals, or by some other means, to
cylindrical cavity. The outlet tube from the cylindrical cavity locate the collected sample spots for subsequent analysis. A
passes at a right angle into the pump compartment. The lower relay is wired in series with the indexing mechanism to turn off
block shall be spring-loaded with a total force of 1.36 kg (3 lb) the vacuum pump during tape transport.
against the lower surface of the upper block. The surfaces of 7.1.2.4 Interval Timer, used to provide desired sampling
thetwoblocksshallbemachinedflattoensureatightseal.The times.
FIG. 2 Schematic Drawing of Double Paper Tape Sampler
D3266
7.1.2.5 Carbon-Vane Vacuum Pump, to sample air, of nomi- 8. Reagents and Materials
nal 30 L/min (1 ft /min) free-air capacity. This provides a
8.1 Purity of Reagents—All reagents shall conform to the
sampling rate through two tapes of about 15 L/min (0.5 ft
specifications of the Committee on Analytical Reagents of the
3/min). Exhaust air from the pump is passed through a soda
American Chemical Society, where such specifications are
lime-glass wool filter (S ) and the filtered air is used to
available.
p
pressurizethefrontcompartmentandpreventcontaminationby
8.2 Purity of Water—Water shall be Grade II Reagent
fluorides from the ambient air. conforming to Specification D 1193. Additionally, the water
used in the sampling and analytical procedures shall be
demonstrated by testing with a specific ion electrode or by
concentration and photometric analysis to contain less than
0.005 µg/mm of fluoride.
8.3 Chemicallytreatedmediumretentivefilterpapertape38
mm (1.5 in.) wide shall be used as the prefilter.
8.4 Chemicallytreatedsoftopenfilterpaper38mm(1.5in.)
wide shall be used to remove acidic gaseous fluorides.
8.5 Citric Acid, Alcoholic, Solution (0.1 M)—Dissolve
4.203 g of citric acid monohydrate in 200 mL of 95% ethyl
alcohol.
8.6 Sodium Hydroxide, Alcoholic Glycerin Solution (0.5
N)—Dissolve 4.000 g of NaOH pellets in 200 mL of 95 %
ethyl alcohol containing 5 % glycerol.
8.7 Total Ionic Strength Adjustment Buffer (TISAB)—Add
57 mL of glacial acetic acid, 58 g of NaCl and 4.0 g of CDTA
((1,2-cyclohexylenedinitrilo)tetraacetic acid) to 500 mL of
distilled water. Stir and add 5 N NaOH solution (8.11) slowly
until pH is between 5.0 and 5.5. Cool and dilute to 1 L.
8.8 TISAB (1 + 1)—Dilute the full strength TISAB (8.7)
1 + 1 with an equal amount of reagent water.
FIG. 3 Inlet Flow Calibration Schematic
8.9 Sulfuric Acid (1.0 N)—Add 28.0 mL of concentrated
H SO (sp gr 1.84) to 250 mL of reagent water in a 1-L
2 4
volumetric flask. Swirl to mix, cool, and dilute to 1 L with
reagent water. Mix thoroughly.
7.1.2.6 Sample Flow Adjustment Valve—An inline needle
8.10 Sodium Hydroxide Solution (1.0 N)—Dissolve 40.0 g
valve, V .
of NaOH in 250 mLof reagent water in a 1000-mLvolumetric
7.1.2.7 Flow Indicator—0–30 L/min (0–1 ft /min) M .
flask. Swirl to mix, cool, and dilute to 1000 mL with reagent
7.1.2.8 Paper Tape—38 mm (1.5 in.) wide, appropriately
water. Mix thoroughly.
treated chemically (10.1).
8.11 Sodium Hydroxide Solution (5.0 N) Dissolve 200.0 g
of NaOH in a 1-L volumetric flask. Swirl to mix, cool, and
7.1.2.9 Provision shall be made for manual override of the
dilute to 1 L with water. Mix thoroughly.
tape transport mechanism.
8.12 Hydrogen Fluoride Permeation Tube— 200 ng/min at
7.1.2.10 All fittings shall be constructed of TFE-
35°C is satisfactory.
fluorocarbon.
7.2 Calibration Equipment—See Fig. 3.
9. Sampling
7.2.1 InletCalibrationAdapter—Toconnecthosefromflow
9.1 See Practice D 1357 for general sampling guidelines.
calibration equipment to sampler inlet.
9.2 Carefully align the sample block assembly to minimize
7.2.2 Flow Meter—M , 0–30 L/min (0–1 ft /min), cali-
leakage.
brated in accordance with Practice D 3195.
7.2.3 Wet Testmeter—M , calibrated in accordance with
Test Methods D 1071.
Reagent Chemicals, American Chemical Society Specifications, American
7.3 HF Permeation Tube Calibrator— A permeation tube Chemical Society, Washington, DC. For suggestions on the testing of reagents not
listed by the American Chemical Society, see Analar Standards for Laboratory
device, modified as described in Footnote 10. See also Practice
Chemicals, BDH Ltd., Poole, Dorset, U.K., and the United States Pharmacopeia
D 3609. All components of the calibrator that come into
and National Formulary, U.S. Pharmacopeial Convention, Inc. (USPC), Rockville,
contact with HF shall be constructed of TFE-fluorocarbon. MD.
D3266
9.3 Adjust temperature controller for a temperature of 54°C 12. Procedure for Obtaining Tape “Blank Values”
(130°F).
12.1 Blank on Reagents—About 50 % of the treated tapes
9.4 Adjust flow rate to 15 L/min (0.5 ft /min).
should be checked for fluoride levels by preparing and analyz
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.