ASTM D1347-72(1995)
(Test Method)Standard Test Methods for Methylcellulose (Withdrawn 2003)
Standard Test Methods for Methylcellulose (Withdrawn 2003)
SCOPE
1.1 These test methods cover the testing of methylcellulose.
1.2 The test methods appear in the following order: Sections Moisture 4 and 5 Ash-as Sulfate 6 to 8 Chlorides-as Sodium Chloride 9 to 11 Alkalinity-as Na2CO3 12 to 14 Iron 15 to 19 Heavy Metals 20 to 22 Methoxyl Content 23 to 26 Viscosity: Water-Soluble Methylcellulose 27 to 29 Alkali-Soluble Methylcellulose 30 to 31 pH 32 Solids 33 to 34 Density 35 to 39
1.3 This standard does not purport to address the safety problems, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.> For a specific hazard statement, see Note 1.
WITHDRAWN RATIONALE
These test methods cover the testing of methylcellulose.
Formerly under the jurisdiction of Committee D01 on Paint and Related Coatings, Materials, and Applications, these test methods were withdrawn in December 2003 in accordance with section 10.6.3.1 of the Regulations Governing ASTM Technical Committees, which requires that standards shall be updated by the end of the eighth year since the last approval date.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Please contact ASTM International (www.astm.org) for the latest information.
Designation: D 1347 – 72 (Reapproved 1995)
Standard Test Methods for
Methylcellulose
This standard is issued under the fixed designation D 1347; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope vided it is first ascertained that the reagent is of sufficiently
high purity to permit its use without lessening the accuracy of
1.1 These test methods cover the testing of methylcellulose.
the determination.
1.2 The test methods appear in the following order:
3.2 Unless otherwise indicated, references to water shall be
Sections
understood to mean distilled water.
Moisture 4 and 5
Ash—as Sulfate 6-8
Chlorides—as Sodium Chloride 9-11
MOISTURE
Alkalinity—as Na CO 12-14
2 3
Iron 15-19
4. Procedure
Heavy Metals 20-22
Methoxyl Content 23-26
4.1 Transfer 2 to5gofthe sample, weighed to the nearest
Viscosity:
0.01 g, to a tared dish (fitted with a lid) and dry it for 3 h in an
Water-Soluble Methylcellulose 27-29
oven at 105 6 3°C. Remove the dish from the oven, cover it
Alkali-Soluble Methylcellulose 30 and 31
pH 32
with a lid, cool in a desiccator, and weigh.
Solids 33 and 34
Density 35-39
5. Calculation
1.3 This standard does not purport to address the safety
5.1 Calculate the percent moisture, M, as follows:
concerns, if any, associated with its use. It is the responsibility
M 5 A/B! 3 100 (1)
~
of the user of this standard to establish appropriate safety and
health practices and determine the applicability of regulatory
where:
limitations prior to use. For a specific hazard statement, see
A = mass loss on heating, g, and
Note 1. B = sample used, g.
2. Referenced Documents
ASH—AS SULFATE
2.1 ASTM Standards:
6. Reagent
D96 Test Methods for Water and Sediment in Crude Oil by
Centrifuge Method (Field Procedure)
6.1 Sulfuric Acid (sp gr 1.84)—Concentrated sulfuric acid
(H SO ).
2 4
3. Purity of Reagents
7. Procedure
3.1 Reagent grade chemicals shall be used in all tests.
Unless otherwise indicated, it is intended that all reagents shall
7.1 Weigh, to the nearest 0.01 g, about2gofthe sample
conform to the specifications of the Committee on Analytical
(previously dried for ⁄2 h at 105°C) and transfer it to a tared
Reagents of the American Chemical Society, where such
platinum crucible. Place it in a muffle furnace at 575 6 25°C
specifications are available. Other grades may be used, pro-
for approximately ⁄2 h, to char the organic material.
7.2 Cool the crucible and add 1 mL of H SO so that it
2 4
completely wets the charred residue. Then cautiously heat it
ThesetestmethodsareunderthejurisdictionofASTMCommitteeD-1onPaint
over a small flame to dense white fumes. Place the crucible in
and Related Coatings, Materials, and Applications, and are the direct responsibility
of Subcommittee D01.36 on Cellulose and Cellulose Derivatives.
a muffle furnace at 575 6 25°C and leave it there until all signs
Current edition approved Feb. 9, 1972. Published March 1972. Originally
of carbon are gone (approximately 1 h). Transfer the specimen
published as D 1347 – 54 T. Last previous edition D 1347 – 64.
2 to a desiccator until cool, then weigh.
Annual Book of ASTM Standards, Vol 05.01.
Reagent Chemicals, American Chemical Society Specifications, American
8. Calculation
Chemical Society, Washington, DC. For suggestions on the testing of reagents not
listed by the American Chemical Society, see Analar Standards for Laboratory
8.1 Calculate the percent of ash, C, as follows:
Chemicals, BDH Ltd., Poole, Dorset, U.K., and the United States Pharmacopeia
and National Formulary, U.S. Pharmaceutical Convention, Inc. (USPC), Rockville, C 5 ~A/B! 3 100 (2)
MD.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Please contact ASTM International (www.astm.org) for the latest information.
D 1347 – 72 (1995)
12.2 Sulfuric Acid, Standard (0.01 N)—Prepare and stan-
where:
dardize a 0.01 N solution of sulfuric acid (H SO ).
A = ash, g, and
2 4
B = sample used, g.
13. Procedure
CHLORIDES—AS SODIUM CHLORIDE
13.1 Weigh, to the nearest 0.01 g, about 1.0 g of the sample
(previously dried for ⁄2 h at 100 to 105°C) and transfer it to a
9. Reagents
500-mL wide-mouth Erlenmeyer flask. Add 250 mL of hot
9.1 Ferric Alum Indicator Solution—Add 100 g of ferric
water to the flask and swirl it for a few minutes, then cool to
aluminum sulfate (Fe SO ) ·(NH ) SO · 24H O) to 250 mLof dissolve.
2 4 3 4 2 4 2
water.HeatittoboilingandaddHNO (spgr1.42)slowlyuntil
13.2 Add 4 drops of methyl purple indicator to the flask
theredcolorisremoved.Thiswillusuallyrequireabout6to15 solutionandtitratetoablue-grayendpointwith0.01NH SO .
2 4
mL of HNO . Filter the solution and store it in a glass bottle.
14. Calculation
9.2 Potassium Thiocyanate, Solution, Standard (0.1 N)—
Dissolve 10 g of potassium thioagonate (KCNS) in 1 L of
14.1 Calculate the percent of alkalinity, D, as anhydrous
water. By means of a pipet, measure 25 mL of 0.1000 N
sodium carbonate (Na CO ) as follows:
2 3
AgNO solution into a 400-mL beaker. Add 100 mL of water,
D 5 [~AB 3 0.053!/C] 3 100 (5)
10 mL of nitric acid (NHO , sp gr 1.42) and 5 mL of ferric
alum indicator solution. Titrate with the KCNS solution, while
where:
stirring, until a faint persistent red color is produced. Calculate A =H SO required for titration of the sample, mL,
2 4
B = normality of the H SO , and
the normality of the KCNS solution, N, as follows:
2 4
C = sample used, g.
N 5 ~A/B! 3 0.1 (3)
IRON
where:
A = 0.100 N AgNO solution added, mL, and
15. Apparatus
B = KCNS solution required for the titration, mL.
9.3 Silver Nitrate, Solution, Standard (0.1 N)—Grind silver 15.1 Photometer—Any photoelectric filter photometer or
spectrophotometer suitable for measurements at 430 nm.
nitrate (AgNO ) crystals fine enough to pass through a 850-µm
(No. 20) sieve, and then dry for2hat110°C. Prepare a 0.1000 15.2 KjeldahlFlasks,calibratedtocontain30mL,andmade
of heat- and chemical-resistant glass.
N solution by dissolving 16.989 g of dry AgNO in chloride-
free water and diluting it to 1 L in a volumetric flask.
16. Reagents
10. Procedure
16.1 Ammonium Hydroxide (sp gr 0.90)—Concentrated am-
monium hydroxide (NH OH).
10.1 Weigh, to the nearest 0.01 g, about 1.0 g of the sample
16.2 Buffer Solution—Dissolve 20 g of sodium bicarbonate
(previously dried for ⁄2 h at 100 to 105°C) and transfer to a
(NaHCO ) and 10 g of sodium carbonate (Na CO ) in water
500-mL wide-mouth Erlenmeyer flask. Add 250 mL of hot
3 2 3
water to the flask and swirl it for a few minutes, then cool to and dilute to 1 L.
16.3 Disodium-1,2-Dihydroxybenzene-3,5-Disulfonate
dissolve.
10.2 Add 5 mL of 0.1000 N AgNO solution and 5 mL of Solution —Prepare an aqueous solution containing 25 g/L.
16.4 Hydrogen Peroxide (30 %)—Concentrated hydrogen
ferric alum indicator solution, and then back-titrate with 0.1 N
KCNS solution to the first appearance of a faint pink color. peroxide (H O ).
2 2
16.5 Iron, Solution, Standard (0.0001 g Fe/mL)—Dissolve
11. Calculation
0.01 g of iron powder containing not less than 99.9 % Fe in
hydrochloric acid (HCl, sp gr 1.19). Oxidize the solution with
11.1 Calculate the percent of chlorides, C, as sodium
bromine water and expel the excess by boiling. Dilute to 1 Lin
chloride (NaCl) as follows:
a volumetric flask.
C 5 ~@~AB 2 CD! 3 0.0585]/E! 3 100 (4)
16.6 Phenolphthalein Indicator Solution.
16.7 Sulfuric Acid (sp gr 1.84)—Concentrated sulfuric acid
where:
A = AgNO solution added, mL, (H SO ).
2 4
B = normality of the AgNO solution, 16.8 Sulfuric Acid (1 + 4)—Carefully mix 1 volume of
C = KCNS solution required to back-titrate the excess H SO (sp gr 1.84) with 4 volumes of water, adding the H SO
2 4 2 4
AgNO , mL,
gradually while mixing.
D = normality of the KCNS solution, and
E = sample used, g. 17. Preparation of Calibration Curve
17.1 FollowingtheproceduregiveninSection18,andusing
ALKALINITY—AS SODIUM CARBONATE,
varied amounts of the standard iron solution prepared in
ANHYDROUS
12. Reagents
A suitable prepared solution of this reagent, known as Tiron, is available from
12.1 Methyl Purple Indicator Solution. the La Motte Chemical Products Co., Chestertown, MD.
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Please contact ASTM International (www.astm.org) for the latest information.
D 1347 – 72 (1995)
accordance with 16.1, prepare a calibration curve showing iron 21.7 Phenolphthalein Indicator Solution.
content in parts per million and the corresponding photometer
22. Procedure
readings.
22.1 Add 5 mLof HCl (1 + 3) to the residue in the platinum
18. Procedure
crucible that was used in the sulfate ash determination (Sec-
tions 6 and 7). Digest the residue by slowly boiling for a few
18.1 Weigh approximately2gofthe sample of methylcel-
minutes over a small flame. Transfer the contents of the
lulose to the nearest 0.01 g, and transfer by means of a funnel
crucible to a 50-mL volumetric flask, using about 25 mL of
to a Kjeldahl flask. Place the flask at a 20° angle in a furnace
water to rinse the crucible. Neutralize with NH OH (1 + 5) to
at 600°C, or on a microdigestion rack equipped with electric
a phenolphthalein end point and dilute to 50 mL.
heating elements, and heat until some charring of the methyl-
22.2 Transfer a 25-mL aliquot of the solution to a 50-mL
cellulose has taken place. (Care must be taken not to char too
Nessler tube, and add 2 mL of acetic acid (6 + 94) and 10 mL
much.) Remove and allow to cool.
of a saturated solution of H S. Mix, allow to stand for 10 min,
18.2 Add3mLofconcentratedH SO totheflask.Placethe
2 4
and compare with a standard lead solution to which H S has
flask on the digestion rack and digest. Cool, and add H O
2 2
been added.
dropwise until the solution is clear. Heat over a Meker burner
22.3 Report the lead content in parts per million.
to a volume of 2 mL. Cool, and wash the sides of the flask with
water. Add 3 drops of phenolphthalein indicator solution. Add
METHOXYL CONTENT
NH OH to a red end point. Wash the neck of the flask. The
solution should be clear and not greater than 20 mLin volume.
23. Apparatus
18.3 Add 2 mL of the color-forming solution described in
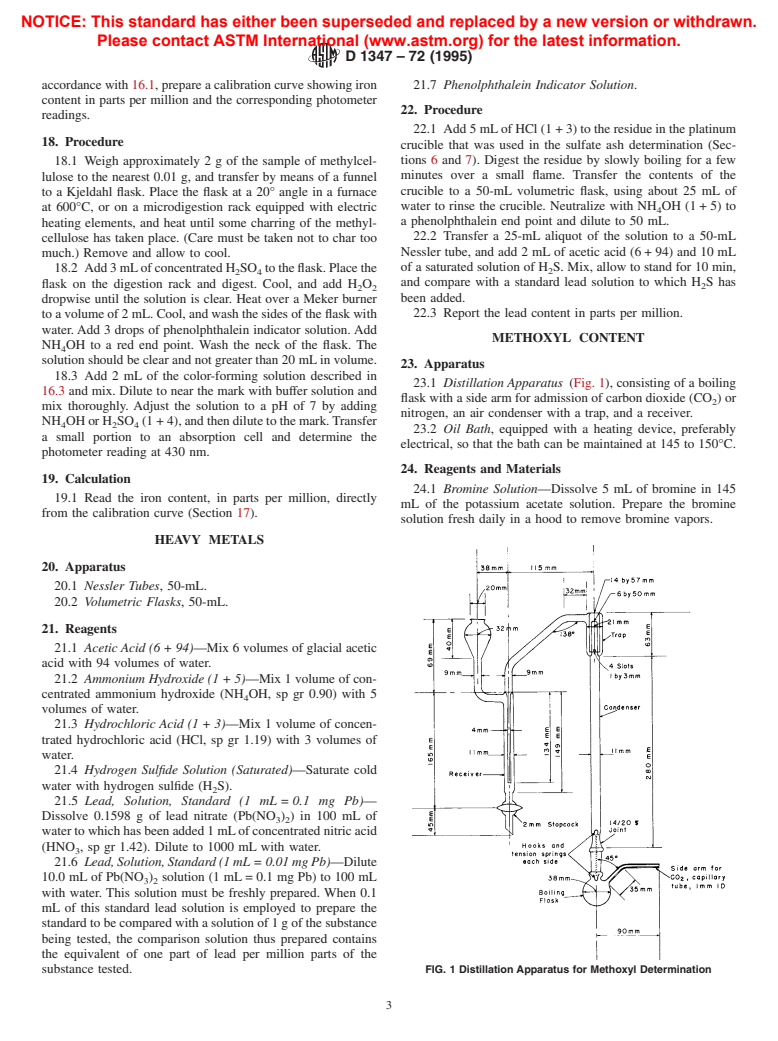
23.1 Distillation Apparatus (Fig. 1), consisting of a boiling
16.3 and mix. Dilute to near the mark with buffer solution and
flask with a side arm for admission of carbon dioxide (CO )or
mix thoroughly. Adjust the solution to a pH of 7 by adding
nitrogen, an air condenser with a trap, and a receiver.
NH OHorH SO (1 + 4),andthendilutetothemark.Transfer
4 2 4
23.2 Oil Bath, equipped with a heating device, preferably
a small portion to an absorption cell and determine the
electrical, so that the bath can be maintained at 145 to 150°C.
photometer reading at 430 nm.
24. Reagents and Materials
19. Calculation
24.1 Bromine Solution—Dissolve 5 mL of bromine in 145
19.1 Read the iron content, in parts per million, directly
mL of the potassium acetate solution. Prepare the bromine
from the calibration curve (Section 17).
solution fresh daily in a hood to remove bromine vapors.
HEAVY METALS
20. Apparatus
20.1 Nessler Tubes, 50-mL.
20.2 Volumetric Flasks, 50-mL.
21. Reagents
21.1 Acetic Acid (6 + 94)—Mix 6 volumes of glacial acetic
acid with 94 volumes of water.
21.2 Ammonium Hydroxide (1 + 5)—Mix 1 volume of con-
centrated ammonium hydroxide (NH OH, sp gr 0.90) with 5
volumes of water.
21.3 Hydrochloric Acid (1 + 3)—Mix 1 volume of concen-
trated hydrochloric acid (HCl, sp gr 1.19) with 3 volumes of
water.
21.4 Hydrogen Sulfide Solution (Saturated)—Saturate cold
water with hydrogen sulfide (H S).
21.5 Lead, Solution, Standard (1 mL = 0.1 mg Pb)—
Dissolve 0.1598 g of lead nitrate (Pb(NO ) ) in 100 mL of
3 2
watertowhichhasbeenadded1mLofconcentratednitricacid
(HNO , sp gr 1.42). Dilute to 1000 mL with water.
21.6 Lead,Solution,Standard(1mL = 0.01mgPb)—Dilute
10.0 mL of Pb(NO ) solution (1 mL = 0.1 mg Pb) to 100 mL
3 2
with water. This solution must be freshly prepared. When 0.1
mL of this standard lead solution is employed to prepare the
standard to be compared with a solution of1gofthe substance
being tested, the comparison solution thus prepared contains
the equivalent of one part of lead per million parts of the
substance tested. FIG. 1 Distillation Apparatus for Methoxyl Determination
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Please contact ASTM International (www.astm.org) for the latest information.
D 1347 – 72 (1995)
24.2 Carbon Dioxide—This may be obtained by the inter- into a 250-mL Erlenmeyer flask. Add2gofKIand50mLof
action of marble and HCl (1 + 1) in a Kipp generator or, H SO (1 + 9) and allow to stand for about 5 min. The flask
2 4
preferably, from a cylinder of the gas equipped with a suitable should be stoppered during the standing period to avoid loss of
needle valve. The CO shall be passed through a bubble iodine. Titrate the liberated iodine with the Na S O solution,
2 2 2 3
counter and a dry trap, and then through a pressure regulator using starch indicator solution near the end point. At the end
consisting of a glass tee whose vertical arm extends almost to point, the blue color of the starch indicator will be destroyed,
the bottom of a 254-mm column of water. A screw clamp is leaving the pale green color of the chromate ion.The normality
attached to the thin-walled rubber tubing connecting the of the Na S O solution should be checked at least once a
2 2 3
horizontal arm of the tee with the boiling flask. This arrange- week. Calculate the normality of the Na S O solution, N,as
2 2 3
ment permits regulation of the flow of gas and allows any follows:
excess gas to escape. Nitrogen may be used in place of CO .
N 5 A/B! 3 0.1 (6)
~
24.3 Formic Acid (HCOOH, 90 %).
where:
24.4 Gelatin Capsules—Gelatin capsules of a suitable size
A = 0.1000 N K Cr O solution added, mL, and
to hold 50 to 60 mg of the dried specimen will be required. 2 2 7
B =Na S O solution required for the titration, mL.
24.5 Hydriodic Acid (57 %, sp gr 1.70)—Hydriodic acid 2 2 3
(HI)formswithwateraconstant-boilingmixture(boilingpoint As an alternative procedure, the Na S O solution may be
2 2 3
126 to 127°C) which contains 57 % HI. The concentration of standardized against 0.1 N iodine solution that has been
HI in the reagent used sho
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.