ASTM F1798-21
(Test Method)Standard Test Method for Evaluating the Static and Fatigue Properties of Interconnection Mechanisms and Subassemblies Used in Spinal Arthrodesis Implants
Standard Test Method for Evaluating the Static and Fatigue Properties of Interconnection Mechanisms and Subassemblies Used in Spinal Arthrodesis Implants
SIGNIFICANCE AND USE
5.1 Spinal implants are generally composed of several components that, when connected together, form a spinal implant construct. Spinal implant constructs are designed to provide some stability to the spine while arthrodesis takes place. This test method outlines standardized evaluations of different interconnection mechanisms to facilitate comparison between different designs. Comparisons must be made cautiously and with careful analysis, taking into account the effects that design differences can have on the loading configurations.
5.2 This test method is used to quantify the static and fatigue properties of different implant interconnection designs. The mechanical tests are conducted in vitro using simplified, unidirectional loads and moments. Fatigue testing in a simulated body fluid or saline may have a fretting, corrosive, or lubricating effect on the interconnection and thereby affect the relative performance of tested devices. Hence, the test environment, whether a simulated body fluid, saline (9 g NaCl per 1000 mL H2O), with a saline drip, or dry, is an important characteristic of the test and must be reported accurately.
5.3 The loading of spinal implant constructs in vivo will, in general, differ from the loading configurations used in this test method. The results obtained here cannot be used directly to predict in vivo performance. However, the results can be used to compare different component designs in terms of relative mechanical parameters.
SCOPE
1.1 This test method covers the measurement of uniaxial static and fatigue strength, and resistance to loosening of the component interconnection mechanisms of spinal arthrodesis implants.
1.2 The purpose of this test method is to provide a means of mechanically characterizing different designs of spinal implant interconnections. Ultimately, the various components and interconnections should be combined for static and fatigue testing of the spinal implant construct. It is not the intention of this test method to address the analysis of spinal implant constructs or subconstructs or to define levels of performance of spinal implants, as insufficient knowledge is available to predict the consequences of the use of particular spinal implant designs.
1.3 This standard defines test methods to measure the strength of spinal implant component interconnections and how to report test results.
1.4 The values stated in SI units are to be regarded as standard, with the exception of angular measurements, which may be reported in terms of either degrees or radians.
1.5 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of regulatory limitations prior to use.
1.6 This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
General Information
Buy Standard
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
Designation: F1798 − 21
Standard Test Method for
Evaluating the Static and Fatigue Properties of
Interconnection Mechanisms and Subassemblies Used in
1
Spinal Arthrodesis Implants
This standard is issued under the fixed designation F1798; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 2. Referenced Documents
2
2.1 ASTM Standards:
1.1 This test method covers the measurement of uniaxial
E4Practices for Force Calibration and Verification of Test-
static and fatigue strength, and resistance to loosening of the
ing Machines
component interconnection mechanisms of spinal arthrodesis
F383Practice for Static Bend and Torsion Testing of In-
implants.
3
tramedullary Rods (Withdrawn 1996)
1.2 Thepurposeofthistestmethodistoprovideameansof
F1717Test Methods for Spinal Implant Constructs in a
mechanicallycharacterizingdifferentdesignsofspinalimplant
Vertebrectomy Model
interconnections. Ultimately, the various components and in-
terconnections should be combined for static and fatigue
3. Terminology
testing of the spinal implant construct. It is not the intention of
3.1 Definitions of Terms Specific to This Standard:
this test method to address the analysis of spinal implant
3.1.1 active length of longitudinal element—the span be-
constructs or subconstructs or to define levels of performance
tween rigid supports (for example, 50 mm is the active length
of spinal implants, as insufficient knowledge is available to
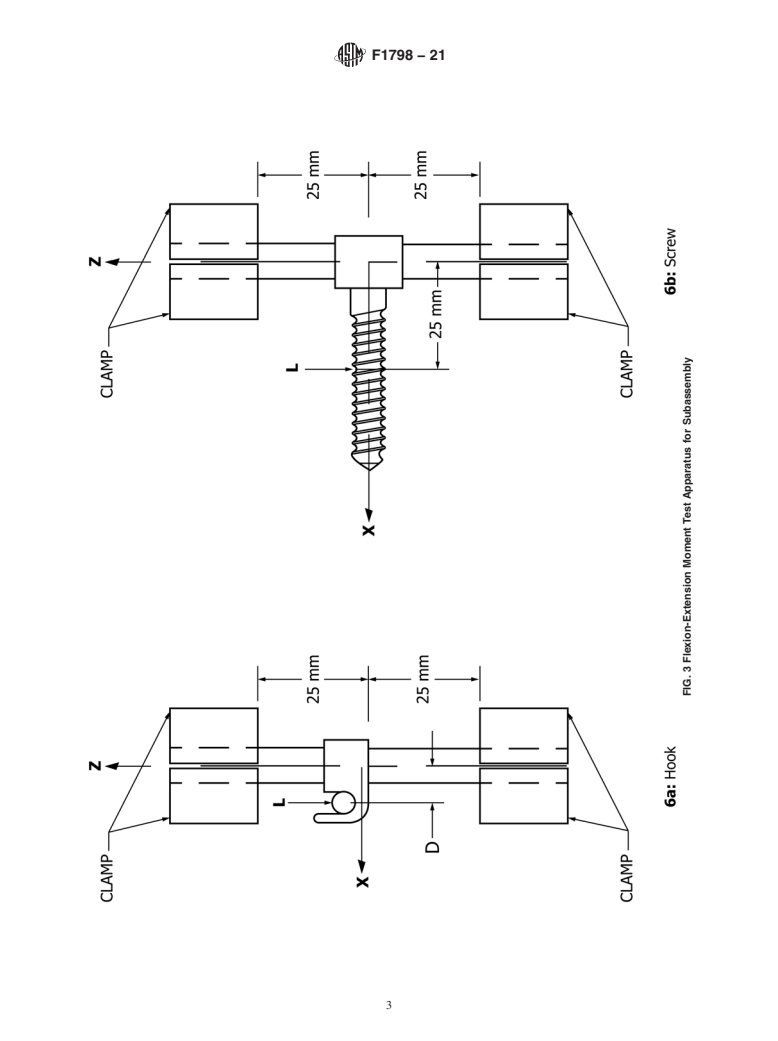
in Fig. 1, Fig. 2, Fig. 3(a), Fig. 3(b), and Fig. 4).
predicttheconsequencesoftheuseofparticularspinalimplant
designs. 3.1.2 global coordinate system—spinal column motion has
six degrees of freedom, having translational motion along, and
1.3 This standard defines test methods to measure the
rotational motion about three axes. The axes are labeled
strength of spinal implant component interconnections and
anterior-posterior orA-P (X), medial-lateral or transverse (Y),
how to report test results.
and caudal-cranial or axial (Z).This coordinate system is right
1.4 The values stated in SI units are to be regarded as
handed with +X in the anterior direction, +Y towards the left
standard, with the exception of angular measurements, which
side of the body, and +Z in the cranial direction. Positive
may be reported in terms of either degrees or radians.
rotations are defined by the right hand rule (see Fig. 5(a)).
1.5 This standard does not purport to address all of the
3.1.3 gripping capacity—the maximum applied load or
safety concerns, if any, associated with its use. It is the
moment across an interconnection mechanism within the first
responsibility of the user of this standard to establish appro-
1.5mmofpermanentdisplacementor5°ofpermanentrotation
priate safety, health, and environmental practices and deter- between the connected components.
mine the applicability of regulatory limitations prior to use.
3.1.4 local coordinate system—thespine’sglobalcoordinate
1.6 This international standard was developed in accor-
system shall be applied locally at the position of the intercon-
dance with internationally recognized principles on standard-
nection. The local direction, z, shall be centered through the
ization established in the Decision on Principles for the
longitudinal element of the x-y plane. The local direction, x,
Development of International Standards, Guides and Recom-
shall be defined as parallel to the axis of a screw or back of a
mendations issued by the World Trade Organization Technical
hook. The local transverse axis, y, shall be parallel to a
Barriers to Trade (TBT) Committee.
transverse element (see Fig. 5(b) and Fig. 5(c)).
1 2
ThistestmethodisunderthejurisdictionofASTMCommitteeF04onMedical For referenced ASTM standards, visit the ASTM website, www.astm.org, or
andSurgicalMaterialsandDevicesandisthedirectresponsibilityofSubcommittee contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
F04.25 on Spinal Devices. Standards volume information, refer to the standard’s Document Summary page on
Current edition approved Oct. 1, 2021. Published October 2021. Originally the ASTM website.
3
approved in 1997. Last previous edition approved in 2013 as F1798–13. DOI: The last approved version of this historical standard is referenced on
10.1520/F1798-21. www.astm.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 -------------
...
This document is not an ASTM standard and is intended only to provide the user of an ASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
Designation: F1798 − 13 F1798 − 21
Standard Test Method for
Evaluating the Static and Fatigue Properties of
Interconnection Mechanisms and Subassemblies Used in
1
Spinal Arthrodesis Implants
This standard is issued under the fixed designation F1798; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope
1.1 This test method covers the measurement of uniaxial static and fatigue strength, and resistance to loosening of the component
interconnection mechanisms of spinal arthrodesis implants.
1.2 The purpose of this test method is to provide a means of mechanically characterizing different designs of spinal implant
interconnections. Ultimately, the various components and interconnections should be combined for static and fatigue testing of the
spinal implant construct. It is not the intention of this test method to address the analysis of spinal implant constructs or
subconstructs or to define levels of performance of spinal implants, as insufficient knowledge is available to predict the
consequences of the use of particular spinal implant designs.
1.3 This test method sets out definitions for use in measuring standard defines test methods to measure the strength of component
interconnections of spinal implants, possible test methods themselves, and the reporting ofspinal implant component intercon-
nections and how to report test results.
1.4 The values stated in SI units are to be regarded as standard, with the exception of angular measurements, which may be
reported in terms of either degrees or radians.
1.5 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility
of the user of this standard to establish appropriate safety safety, health, and healthenvironmental practices and determine the
applicability of regulatory limitations prior to use.
1.6 This international standard was developed in accordance with internationally recognized principles on standardization
established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued
by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
2. Referenced Documents
2
2.1 ASTM Standards:
E4 Practices for Force Calibration and Verification of Testing Machines
3
F383 Practice for Static Bend and Torsion Testing of Intramedullary Rods (Withdrawn 1996)
1
This test method is under the jurisdiction of ASTM Committee F04 on Medical and Surgical Materials and Devices. and is the direct responsibility of Subcommittee
F04.25 on Spinal Devices.
Current edition approved Dec. 1, 2013Oct. 1, 2021. Published February 2014October 2021. Originally approved in 1997. Last previous edition approved in 20082013 as
F1798 – 97F1798 – 13.(2008). DOI: 10.1520/F1798-13. 10.1520/F1798-21.
2
For referenced ASTM standards, visit the ASTM website, www.astm.org, or contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM Standards
volume information, refer to the standard’s Document Summary page on the ASTM website.
3
The last approved version of this historical standard is referenced on www.astm.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
F1798 − 21
F1582F1717 Terminology Relating to Spinal ImplantsTest Methods for Spinal Implant Constructs in a Vertebrectomy Model
3. Terminology
3.1 Definitions of Terms Specific to This Standard:
3.1.1 active length of longitudinal element—the span between rigid supports (for example, 50 mm is the active length in Fig. 1,
Fig. 2, Fig. 3((a),a), Fig. 3((b),b), and Fig. 4.).
3.1.2 global coordinate system—spinal column motion has six degrees of freedom, having translational motion along, and
rotational motion about three axes. The axes are labeled anterior-posterior or a-pA-P (X), medial-lateral or transverse (Y), and
caudal-cranial or axial (Z). This coordinate system is right handed with +X in the anterior direction, +Y towards the left side of
the body, and +Z in the cranial direction. Positive rotations are defined by the right hand rule (see Fig. 5((a)).a)).
3.1.3 gripping capacity—the maximum applied load or moment across an interconnection mechanism within the first 1.5 mm of
perman
...










Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.