ASTM F1839-97
(Specification)Standard Specification for Rigid Polyurethane Foam for Use as a Standard Material for Testing Orthopaedic Devices and Instruments
Standard Specification for Rigid Polyurethane Foam for Use as a Standard Material for Testing Orthopaedic Devices and Instruments
SCOPE
1.1 This specification covers rigid unicellular polyurethane foam for use as a standard material for performing mechanical tests utilizing orthopaedic devices or instruments. The specification is applicable to sheets or blocks of foam, or foam that is made by the user using a two-part liquid mixture.
1.2 This specification covers polyurethane foam material that is used in the laboratory for mechanical testing, as described in 1.1. These materials are not intended for implantation into the human body.
1.3 The foam described herein possesses mechanical properties which are on the order of those reported for human cancellous bone. See Appendix X1 Rationale for further information regarding the appropriateness of using the specified foam as a model for human cancellous bone.
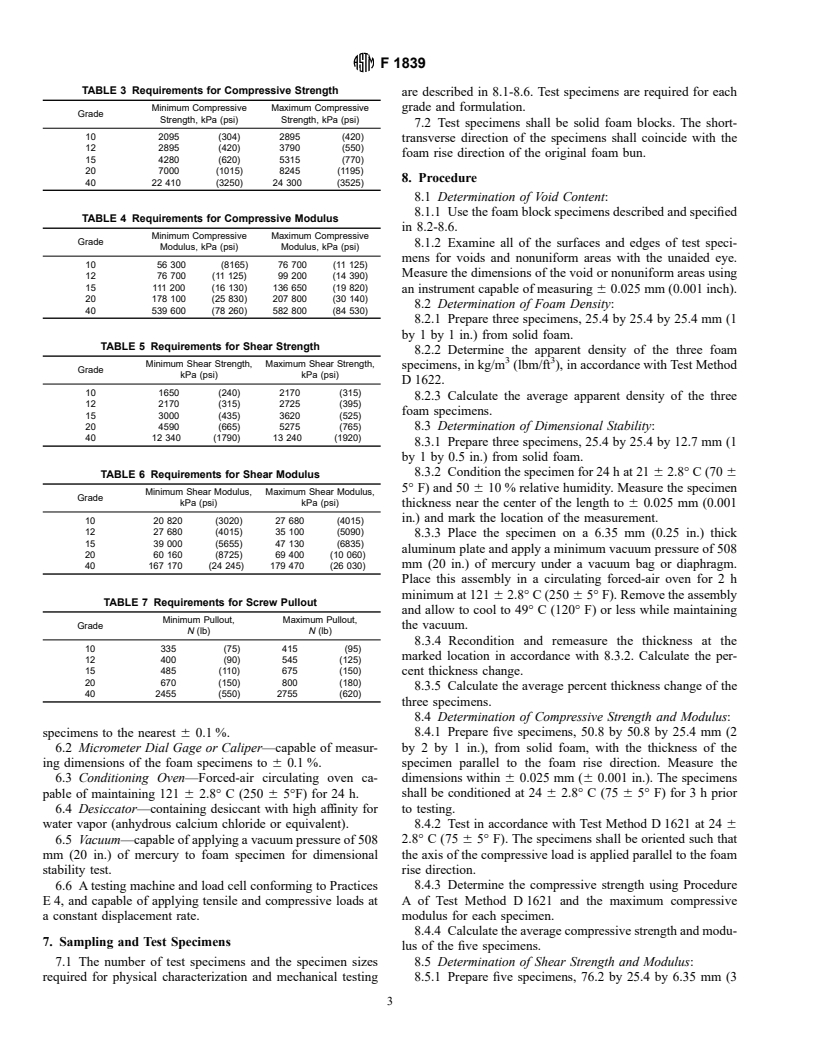
1.4 This specification covers compositional requirements, physical requirements, mechanical requirements, and test methods for rigid polyurethane foam in the solid final form.
1.5 This specification provides qualification criteria for vendor or end-user processes and acceptance criteria for individual material lots.
1.6 This specification provides mechanical properties of five different grades of foam in the solid final form. A foam that does not meet the specified mechanical properties shall be identified as an ungraded foam.
1.7 Unless otherwise indicated, the values stated in SI units are to be regarded as standard. The values in parentheses are given for information only.
1.8 The following precautionary statement pertains to the test method portion only, Section 8, of this specification: This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or
withdrawn. Contact ASTM International (www.astm.org) for the latest information.
Designation: F 1839 – 97
AMERICAN SOCIETY FOR TESTING AND MATERIALS
100 Barr Harbor Dr., West Conshohocken, PA 19428
Reprinted from the Annual Book of ASTM Standards. Copyright ASTM
Standard Specification for
Rigid Polyurethane Foam for Use as a Standard Material for
1
Testing Orthopaedic Devices and Instruments
This standard is issued under the fixed designation F 1839; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope 2. Referenced Documents
1.1 This specification covers rigid unicellular polyurethane 2.1 ASTM Standards:
foam for use as a standard material for performing mechanical C 273 Test Method for Shear Test in Flatwise Plane of Flat
3
tests utilizing orthopaedic devices or instruments. The specifi- Sandwich Constructions or Sandwich Cores
cation is applicable to sheets or blocks of foam, or foam that is D 1621 Test Method for Compressive Properties of Rigid
4
made by the user using a two-part liquid mixture. Cellular Plastics
1.2 This specification covers polyurethane foam material D 1622 Test Method for Apparent Density of Rigid Cellular
4
that is used in the laboratory for mechanical testing, as Plastics
5
described in 1.1. These materials are not intended for implan- E 4 Practices for Load Verification of Testing Machines
tation into the human body. F 117 Test Method for Driving Torque of Medical Bone
6
1.3 The foam described herein possesses mechanical prop- Screws
erties which are on the order of those reported for human F 1691 Test Method for Determining the Axial Pullout of
6
cancellous bone. See Appendix X1 Rationale for further Medical Bone Screws
information regarding the appropriateness of using the speci- 2.2 ISO Standards:
fied foam as a model for human cancellous bone. 5835-1 Implants for Surgery - Metal Bone Screws with
1.4 This specification covers compositional requirements, Hexagonal Driver Connection, Spherical Under Surface of
7
physical requirements, mechanical requirements, and test Head, Asymmetrical Thread - Dimensions
methods for rigid polyurethane foam in the solid final form.
3. Terminology
1.5 This specification provides qualification criteria for
vendor or end-user processes and acceptance criteria for 3.1 Definitions:
3.1.1 final form—the condition of the foam product when
individual material lots.
1.6 This specification provides mechanical properties of five used by the end-user to perform tests of orthopaedic devices or
2
instruments. The condition of the foam product of which all
different grades of foam in the solid final form. A foam that
does not meet the specified mechanical properties shall be physical and mechanical tests required by this specification are
performed.
identified as an ungraded foam.
1.7 Unless otherwise indicated, the values stated in SI units 3.1.1.1 solid—the foam is in a uniform solid form, such as
a slab, plate or block.
are to be regarded as standard. The values in parentheses are
given for information only. 3.1.2 foam rise direction—the nominal direction that the
1.8 The following precautionary statement pertains to the foam rises during the polymerization (“foaming”) process,
either at the suppliers production facilities for the solid
test method portion only, Section 8, of this specification: This
standard does not purport to address all of the safety concerns, supplied foam, or at the end-users facilities for foam produced
from the liquid supplied form. The foam rise direction shall be
if any, associated with its use. It is the responsibility of the user
of this standard to establish appropriate safety and health marked on the foam block or indicated in the shipping
documentation for foam that is supplied in the Solid form.
practices and determine the applicability of regulatory limita-
tions prior to use. 3.1.3 grades—The grade designation refers to the nominal
density of the foam, in its Solid Final form, expressed in units
3 3
of kg/m (lbm/ft ). Five grades of foam have been defined in
this specification. Their nominal densities are given below:
1
This test method is under the jurisdiction of ASTM Committee F-04 on Medical
and Surgical Materials and Devices and is the direct responsibility of Subcommittee
3
F04.21 on Osteosynthesis.
Annual Book of ASTM Standards, Vol 15.03.
4
Current edition approved Dec. 10, 1997. Published May 1998.
Annual Book of ASTM Standards, Vol 08.01.
2
5
General Plastics Manufacturing Company, 4910 Burlington Way, Tacoma
Annual Book of ASTM Standards, Vol 03.01.
6
Washington, 98409, producers of Last-a-Foamt polyurethane foam, has been found
Annual Book of ASTM Standards, Vol 13.01.
7
to be a satisfactory supp
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.