ASTM D2010/D2010M-98(2004)
(Test Method)Standard Test Methods for Evaluation of Total Sulfation Activity in the Atmosphere by the Lead Dioxide Technique
Standard Test Methods for Evaluation of Total Sulfation Activity in the Atmosphere by the Lead Dioxide Technique
SIGNIFICANCE AND USE
Sulfur oxide gases are produced during the combustion of materials containing sulfur. These gases are precursors of atmospheric sulfuric acid, which has been shown to be injurious to living creatures and plants, as well as some inanimate materials such as metals, limestone and sandstone building materials.
Sulfur dioxide is moderately toxic and strongly phytotoxic to many species. Permissible ambient levels of SO2 have been established by law.
When it is necessary to establish whether ambient air concentrations of sulfuric acid precursors, such as sulfur oxides, are present and to comply with legal criteria, manual and automatic monitoring systems specific for the individual sulfur species are used. Likely locations for monitoring sites for the estimation of concentrations and concentration trends over long periods of time can be screened conveniently using the PbO 2 candles or sulfation plates.
Atmospheric corrosion of metallic materials is a function of many weather and atmospheric variables. The effect of specific corrodants, such as SO2, can accelerate the atmospheric corrosion of metals or structures significantly. The PbO2 candle and sulfation plate test methods provide simple techniques to monitor SO2 levels in the atmosphere independently to yield a weighted average result.
The results of these test methods are useful for characterizing atmospheric corrosion test sites regarding the effective average concentrations of SO2 in the atmosphere at these locations.
These test methods are useful for determining microclimatic seasonal and long-term variations in effective average SO2 concentrations.
The results of these test methods may be used in correlations of atmospheric corrosion rates with atmosphere data to determine the sensitivity of the corrosion rate to the SO2 level.
These test methods may also be used with other test methods to characterize the atmosphere at sites at which buildings or other construction are planned in order to determine t...
SCOPE
1.1 These test methods describe the evaluation of the total sulfation activity in the atmosphere. Because of its oxidizing power, lead dioxide (PbO2) converts not only sulfur dioxide (SO2), but other compounds, such as mercaptans and hydrogen sulfide, into sulfate. It fixes sulfur trioxide and sulfuric acid mist present in the atmosphere (see Note 0).
1.2 Test Method A describes the use of a PbO2 candle, and Test Method B describes that of a PbO2 sulfation plate.
1.3 These test methods provide a weighted average effective SO2 level for a 30-day interval.
1.4 The results of these test methods correlate approximately with volumetric SO2 concentrations, although the presence of dew or condensed moisture tends to enhance the capture of SO2 onto the candle or plate.
1.5 The values stated in SI units shall be regarded as the standard. The values given in brackets are for information only and may be approximate.Note 0
It has been shown that the rate constant of the chemical reaction between SO2 and PbO2 is independent of the concentration of SO2 up to levels of 1000 ppm(v), if 15 % or less of the PbO2 has been reduced (). 15 % of the PbO2 is equivalent to 11 to 12 mg of SO2/cm2 per day.
This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use. For specific precautionary statements, see Section .
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation:D2010/D2010M–98 (Reapproved 2004)
Standard Test Methods for
Evaluation of Total Sulfation Activity in the Atmosphere by
the Lead Dioxide Technique
This standard is issued under the fixed designation D2010/D2010M; the number immediately following the designation indicates the
year of original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last
reapproval. A superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
This standard has been approved for use by agencies of the Department of Defense.
1. Scope 2. Referenced Documents
1.1 These test methods describe the evaluation of the total 2.1 ASTM Standards:
sulfation activity in the atmosphere. Because of its oxidizing D516 Test Method for Sulfate Ion in Water
power, lead dioxide (PbO ) converts not only sulfur dioxide D1193 Specification for Reagent Water
(SO ),butothercompounds,suchasmercaptansandhydrogen D1356 Terminology Relating to Sampling and Analysis of
sulfide, into sulfate. It fixes sulfur trioxide and sulfuric acid Atmospheres
mist present in the atmosphere (see Note 1). D1357 Practice for Planning the Sampling of the Ambient
1.2 Test MethodAdescribes the use of a PbO candle, and Atmosphere
Test Method B describes that of a PbO sulfation plate. G91 Practice for Monitoring Atmospheric SO Using the
2 2
1.3 Thesetestmethodsprovideaweightedaverageeffective Sulfation Plate Technique
SO level for a 30-day interval.
3. Terminology
1.4 The results of these test methods correlate approxi-
3.1 Definitions—For definitions of terms used in these test
mately with volumetric SO concentrations, although the
presence of dew or condensed moisture tends to enhance the methods, refer to Terminology D1356.
3.2 Definitions of Terms Specific to This Standard:
capture of SO onto the candle or plate.
1.5 The values stated in SI units shall be regarded as the 3.2.1 sulfation—the process by which sulfur-containing
compounds are oxidized by the action of PbO .
standard.Thevaluesgiveninbracketsareforinformationonly
and may be approximate. 3.2.2 sulfation activity—the capture rate of sulfur-
containing compounds as they are oxidized by PbO under the
NOTE 1—It has been shown that the rate constant of the chemical
conditions of these test methods.
reaction between SO and PbO is independent of the concentration of
2 2
SO up to levels of 1000 ppm(v), if 15% or less of the PbO has been
2 2
4. Summary of Test Methods
3 2
reduced (1). 15% of the PbO is equivalent to 11 to 12 mg of SO /cm
2 2
per day. 4.1 Test Method A—Inert cylinders are coated with PbO
paste and exposed to the atmosphere for an extended period of
1.6 This standard does not purport to address all of the
time, usually one month. Sulfur oxides react chemically with
safety concerns, if any, associated with its use. It is the
the paste, forming lead sulfate (PbSO ) (1-5).
responsibility of the user of this standard to establish appro-
4.2 Test Method B—Sulfation plates consisting of a PbO
priate safety and health practices and determine the applica-
paste in an inverted dish are likewise exposed to the atmo-
bility of regulatory limitations prior to use. For specific
sphere (6).
precautionary statements, see Section 8.
4.3 Test Methods A and B—The cylinders or plates are
returned to a laboratory after the sampling period; the paste is
These test methods are under the jurisdiction of ASTM Committee D22 on
removed and suspended in hot sodium carbonate (Na CO )
2 3
Sampling andAnalysis ofAtmosphere and are the direct responsibility of Subcom-
solution to dissolve the PbSO and convert the sulfate to
mittee D22.03 on Ambient Atmospheres and Source Emissions.
soluble sodium sulfate (Na SO ). The Na SO solution is
CurrenteditionapprovedOctober1,2004.PublishedDecember2004.Originally 2 4 2 4
approved in 1962. Last previous edition approved in 1998 as D2010/D2010M-98.
DOI: 10.1520/D2010_D2010M-98R04.
Test Method B has been adapted from Test Method G91, which is under the
jurisdiction of ASTM Committee G01 on Corrosion of Metals and is the direct For referenced ASTM standards, visit the ASTM website, www.astm.org, or
responsibility of Subcommittee G01.04 on Atmospheric Corrosion. contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Theboldfacenumbersinparenthesesrefertothelistofreferencesattheendof Standards volume information, refer to the standard’s Document Summary page on
this standard. the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
D2010/D2010M–98 (2004)
separated from the PbO slurry by filtration. The sulfate is 6.1.2 Sampling Apparatus—This may be a louvered enclo-
determined by precipitation with barium chloride (BaCl ) (7). sure, such as a cylinder or a rectangular box. If cylindrical, it
shall be not less than 20-cm [8-in.] high and 18-cm [7-in.] in
4.4 The chemistry of the process is illustrated, for the case
of SO , in the following reactions: diameter;ifrectangular,itshallbenotlessthan20by20by20
cm [8 by 8 by 8 in.]. Position the louvers at an angle of p/4
PbO 1 SO → PbSO
2 2 4
(45°) to provide maximum protection from the rain. Construct
PbSO 1 Na CO → Na SO 1 PbCO
4 2 3 2 4 3
the enclosure of an inert material, such as plastic or wood. Do
Na SO 1 BaCl → BaSO ↓ 12 NaCl
2 4 2 4
not coat the enclosure with a lead based paint. The sampling
apparatus shall have provisions to hold the PbO candle in a
vertical position.
5. Significance and Use
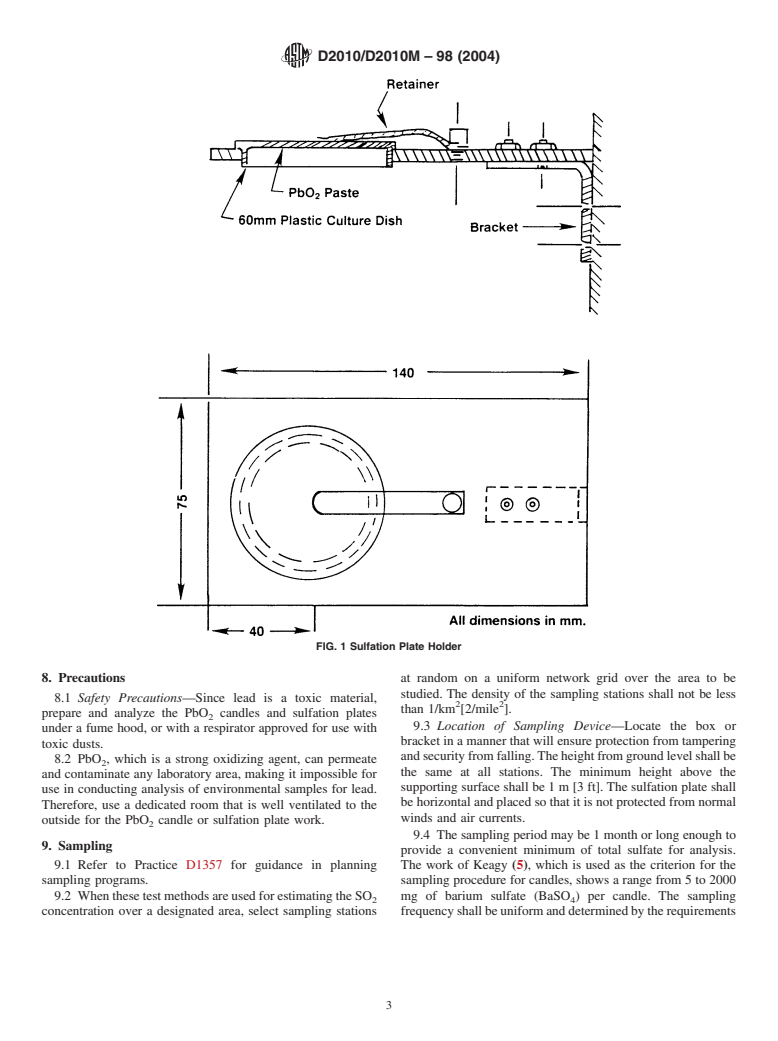
6.2 Test Method B:
5.1 Sulfur oxide gases are produced during the combustion 6.2.1 Sulfation Plate—A polystyrene or polycarbonate cul-
of materials containing sulfur. These gases are precursors of ture (petri) dish, 50 or 60 mm in diameter, containing a filter
atmospheric sulfuric acid, which has been shown to be injuri- paperdisc,coatedwithPbO paste.SeeX1.2forpreparationof
ous to living creatures and plants, as well as some inanimate the sulfation plate.
6.2.2 Bracket, to hold the plates securely in an inverted
materials such as metals, limestone and sandstone building
materials. positionsothatthePbO mixturefacesdownward.Thebracket
design shall include a retaining clip or other provision to hold
5.2 Sulfur dioxide is moderately toxic and strongly phyto-
theplateintheeventofstrongwinds.Theretainerclipmaybe
toxic to many species. Permissible ambient levels of SO have
made from stainless steel, spring bronze, hard aluminum alloy
been established by law.
(3003H19), or other alloys with sufficient strength and atmo-
5.3 When it is necessary to establish whether ambient air
spheric corrosion resistance.Atypical bracket design is shown
concentrations of sulfuric acid precursors, such as sulfur
in Fig. 1.
oxides, are present and to comply with legal criteria, manual
and automatic monitoring systems specific for the individual
7. Reagents and Materials
sulfur species are used. Likely locations for monitoring sites
for the estimation of concentrations and concentration trends
7.1 Purity of Reagents—Reagent grade chemicals shall be
over long periods of time can be screened conveniently using
usedinalltests.Allreagentsshallconformtothespecifications
the PbO candles or sulfation plates.
2 of the Committee on Analytical Reagents of the American
5.4 Atmospheric corrosion of metallic materials is a func-
Chemical Society, except where such reagents are not avail-
tion of many weather and atmospheric variables. The effect of
able.
specific corrodants, such as SO , can accelerate the atmo-
7.2 Purity of Water—References to water shall be under-
spheric corrosion of metals or structures significantly. The
stood to mean reagent water as defined by Type II of Specifi-
PbO candle and sulfation plate test methods provide simple
cation D1193.
techniques to monitor SO levels in the atmosphere indepen-
7.3 Acetone—Reagent grade.
dently to yield a weighted average result.
7.4 Barium Chloride Solution (50 g/L)—Dissolve 59 g of
5.5 The results of these test methods are useful for charac- bariumchloridedihydrate(BaCl 32H O)inwateranddilute
2 2
terizingatmosphericcorrosiontestsitesregardingtheeffective
to1L.
average concentrations of SO in the atmosphere at these 7.5 Ethyl Alcohol (95%).
locations.
7.6 Gum Tragacanth, powdered.
5.6 These test methods are useful for determining microcli-
7.7 Hydrochloric Acid (sp gr 1.19)—Concentrated hydro-
matic seasonal and long-term variations in effective average chloric acid (HCl).
SO concentrations.
7.8 Hydrochloric Acid (2 N)—Dilute 171 mL of concen-
5.7 The results of these test methods may be used in trated HCl to 1 L.
correlations of atmospheric corrosion rates with atmosphere 7.9 Hydrochloric Acid (0.05 N)—Dilute 25 mL of 2 N HCl
datatodeterminethesensitivityofthecorrosionratetotheSO to1L.
level. 7.10 Lead Dioxide (Powdered)—PbO ofthehighestpurity.
7.11 Sodium Carbonate Solution (83.3 g/L)—Dissolve 83.3
5.8 These test methods may also be used with other test
methods to characterize the atmosphere at sites at which gofanhydroussodiumcarbonate(Na CO )inwateranddilute
2 3
to1L.
buildings or other construction are planned in order to deter-
mine the extent of protective measures required for the 7.12 Methyl Orange Indicator (0.1 %)—Dissolve100mgin
water and dilute to 100 mL.
materials of construction.
6. Apparatus
6.1 Test Method A:
Reagent Chemicals, American Chemical Society Specifications, American
Chemical Society, Washington, DC. For suggestions on the testing of reagents not
6.1.1 Lead Dioxide Candle—An inert cylinder with a sur-
2 listed by the American Chemical Society, see Analar Standards for Laboratory
face area of approximately 100 cm , covered with a fabric and
Chemicals, BDH Ltd., Poole, Dorset, U.K., and the United States Pharmacopeia
coated with PbO paste. See Appendix X1 for preparation of
2 and National Formulary, U.S. Pharmacopeial Convention, Inc. (USPC), Rockville,
the candle. MD.
D2010/D2010M–98 (2004)
FIG. 1 Sulfation Plate Holder
8. Precautions at random on a uniform network grid over the area to be
studied. The density of the sampling stations shall not be less
8.1 Safety Precautions—Since lead is a toxic material,
2 2
than 1/km [2/mile ].
prepare and analyze the PbO candles and sulfation plates
9.3 Location of Sampling Device—Locate the box or
under a fume hood, or with a respirator approved for use with
bracketinamannerthatwillensureprotectionfromtampering
toxic dusts.
andsecurityfromfalling.Theheightfromgroundlevelshallbe
8.2 PbO , which is a strong oxidizing agent, can permeate
the same at all stations. The minimum height above the
and contaminate any laboratory area, making it impossible for
supporting surface shall be 1 m [3 ft].The sulfation plate shall
use in conducting analysis of environmental samples for lead.
behorizontalandplacedsothatitisnotprotectedfromnormal
Therefore, use a dedicated room that is well ventilated to the
winds and air currents.
outside for the PbO candle or sulfation plate work.
9.4 The sampling period may be 1 month or long enough to
9. Sampling
provide a convenient minimum of total sulfate for analysis.
9.1 Refer to Practice D1357 for guidance in planning The work of Keagy (5), which is used as the criterion for the
sampling programs. sampling procedure for candles, shows a range from 5 to 2000
9.2 WhenthesetestmethodsareusedforestimatingtheSO mg of barium sulfate (BaSO ) per candle. The sampling
2 4
concentration over a designated area, select sampling stations frequencyshallbeuniformanddeterminedbytherequirements
D2010/D2010M–98 (2004)
of the survey. Monthly, bimonthly, and seasonal sampling 12.1.1.1 The standard deviation, S , for the reproducibility
b
periods have been shown to provide consistent and reliable of total sulfation activity measurements by different laborato-
data (5). ries ranging from 0.00178 to 0.01371 mg/cm 3day may be
expressed by the following equation:
10. Analytical Procedure
½
S 50.0136 M (2)
b
10.1 Return the candles or plates to containers that can be
sealed from contamination at the end of the sampling period.
where:
10.2 Test Method A, Treatment of Candles—Measure the S and M = mg/cm 3day.
b
surface area of the candle. Separate the impregnated cloth
12.1.1.2 The standard deviation, S , for replicate measure-
w
surface from the cylinder, using a spatula or knife point, if ments of total sulfation activity ranging from 0.00178 to
necessary. The fabric may be cut into smaller pieces. Transfer
0.01371 mg/cm 3day by the same laboratory (repeatability)
thePbO -coveredfabrictoa250-mLbeakercontaining60mL may be expressed by the following equation:
of 83.3-g/L solution of Na CO (7.11). Soak the immersed
2 3 ½
S 50.00504 M (3)
w
pieces for 3 h, with occasional stirring. Cover the beakers, and
simmer the mixtures gently on a water bath plate for 30 min,
where:
taking care to minimize water evaporation in order to maintain S and M = mg/cm 3day.
w
an approximately constant volume. Filter the beaker contents
12.1.2 Theaverageresultsoftheanalysisofspikedsamples
through a fast filter paper, with appropriate washings, and (8) indicates that the determination of sulfate by Test Method
adjustthefiltratewith2NHCl(7.8)toapHrangeof3.0to4.0,
D516 can be performed with a recovery of 98%.
using methyl orange as the indicator (7.12). Exercise care to 12.1.2.1 The standard deviation of the percent of sulfate
prevent any loss of sample by foaming, particularly when the
spikerecoveryofthesulfateanalysisstepis10%forbetween-
point of neutralization is approached. laboratory measurements and 21% for within-laboratory mea-
10.3 Test Method B—Remove the contents of the sulfation
surements.
plate to a 250-mLbeaker, and add 12 mLof 83.3 g/LNa CO
12.2 Test Method B (9):
2 3
solution (7.11). Cover the beaker, and proceed as described in
12.2.1 The standard deviation of replicate plates run under
10.2.
the same exposure conditions for a single laboratory has been
10.4 Determination of Sulfate as Barium Sulfate—
found to be related to the mean sulfation level by the equation
Determine the sulfate ion in accordance with the gravimetric
given below:
test method (Test MethodA) in Test Method D516. The rapid
s50.0790 m (4)
avg
addition of a boiling solution of BaCl (7.4) to a gently boiling
where:
solution of the sulfate in 0.05 N HCl (7.9) will yield a granular
s = standard deviation in mg SO /m 3day, and
and easily filterable BaSO precipitate.
m = mean net SO capture rate in mg SO /m 3day
10.5 Determine the sulfate in the unexposed (blank) candle avg 2 2
based on 10 runs with six or more plates per run.
or p
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.